Abstract
The spindle checkpoint prevents anaphase onset until completion of mitotic spindle assembly by restraining activation of the ubiquitin ligase anaphase-promoting complex/cyclosome–Cdc20 (APC/CCdc20). We show that the spindle checkpoint requires mitotic cyclin-dependent kinase (cdk) activity. Inhibiting cdk activity overrides checkpoint-dependent arrest in Xenopus egg extracts and human cells. Following inhibition, the interaction between APC/C and Cdc20 transiently increases while the inhibitory checkpoint protein Mad2 dissociates from Cdc20. Cdk inhibition also overcomes Mad2-induced mitotic arrest. In addition, in vitro cdk1-phosphorylated Cdc20 interacts with Mad2 rather than APC/ C. Thus, cdk activity is required to restrain APC/CCdc20 activation until completion of spindle assembly.
Keywords: Checkpoint, cdk, spindle assembly, APC/C
At the end of mitosis, activation of the ubiquitin ligase anaphase-promoting complex/cyclosome (APC/C) in association with its coactivator Cdc20 (APC/CCdc20) initiates proteolysis of the anaphase inhibitor securin and of mitotic cyclins leading to anaphase onset (Peters 2002). From telophase to G1, in yeast and somatic mammalian cells, APC/C associates with a different coactivator, Cdh1 (APC/CCdh1; Peters 2002). In early embryonic cell cycles, that lack G1, Cdh1 is not expressed, and APC/C activity depends only on Cdc20 (Lorca et al. 1998; Peters 2002). The spindle checkpoint, a safeguard mechanism that prevents defects in chromosome segregation, inhibits anaphase onset until the mitotic spindle is correctly assembled (Peters 2002). The spindle checkpoint blocks APC/CCdc20 activation.
Phosphorylation of key elements may have a role in timing the events that lead to mitosis exit and in regulating the spindle checkpoint function. Phosphorylation of APC/C subunits by cdk and Polo-like kinase appears to help APC/C activity. However, cdk activity blocks APC/CCdh1 activation (Peters 2002). In addition, recent evidence indicates that cdk activity is required for the efficient function of the spindle checkpoint protein Bub1p in fission yeast (Yamaguchi et al. 2003). Whether cdk activity has a role in the checkpoint-dependent control of APC/CCdc20 activation in higher eukaryotes is unclear (Kramer et al. 2000, Yudkovsky et al. 2000). We have now used cycling Xenopus egg extracts and human cells to investigate the role for mitotic cdk activity in the spindle checkpoint function.
The spindle checkpoint does not inhibit cyclin A degradation; thus, cdk1 activity is sustained by cyclin B under checkpoint conditions (Peters 2002). In somatic cells, cyclin E is degraded during S phase and its abundance has been reported to be very low in mitosis (Dulic et al. 1992). Because of the low abundance of its partners, cyclin A and cyclin E, cdk2 may not have a crucial role for mitotic arrest induced by the spindle checkpoint in somatic cells (Peters 2002). In Xenopus embryos and egg extracts cyclin E is not degraded during S phase, and cyclin E–cdk2 activity oscillates during mitosis, regulating several aspects of this phase of the cell cycle (Guadagno and Newport 1996; D'Angiolella et al. 2001; Tunquist et al. 2002). Cyclin E–cdk2 appears required to stimulate the cdk1 activation pathways at the onset of mitosis (Guadagno and Newport 1996). In addition, we and others have recently shown that cyclin B degradation depends on the proteolysis-independent down-regulation of cyclin E–cdk2 activity at the end of mitosis, and that constitutively active cyclin E–cdk2 induces checkpoint-like mitotic arrest (D'Angiolella et al. 2001; Tunquist et al. 2002; Tunquist and Maller 2003).
Results and Discussion
Cdk activity is required to prevent activation of the cyclin degradation pathway in checkpoint-arrested egg extracts
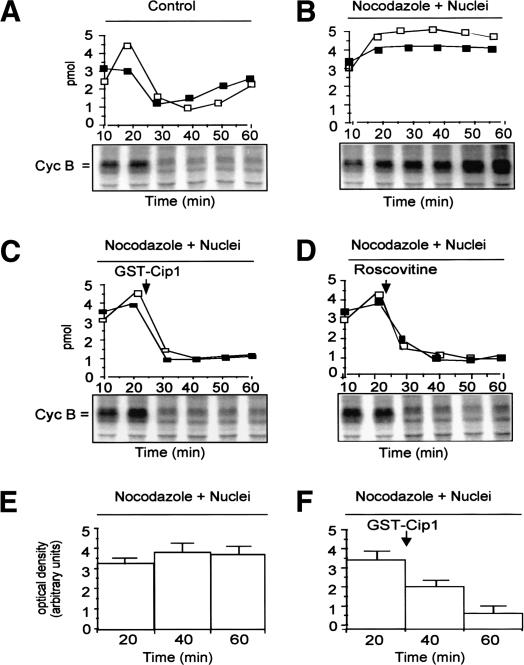
The spindle checkpoint can be activated in Xenopus egg extract by adding large amounts of demembranated sperm nuclei in the presence of the microtubule inhibitor nocodazole (Minshull et al. 1994). To study the role for mitotic cdk activity in the spindle checkpoint, we first asked whether the activity of cdk2 was stabilized, along with that of cdk1, under checkpoint conditions. We monitored the kinase activity of M-phase promoting factor (MPF; the cyclin B–cdk1 complex) and cyclin B stability, as well as cdk2 activity during incubation at 23°C of control extracts and extracts in which the spindle checkpoint was activated. In the control sample, MPF activity and cyclin B abundance increased until 20 min and fell by 30 min (Fig. 1A). Cdk2 activity was higher during mitosis (Fig. 1A, 20 min) and declined thereafter (D'Angiolella et al. 2001). In the checkpoint sample, stabilization of cyclin B and MPF activity was accompanied by stabilization of cdk2 activity (Fig. 1B). To determine the relevance of sustained cdk2 activity for the checkpoint, we added to a portion of checkpoint-arrested extract the cdk inhibitor protein p21/Cip1/Waf1 (as a glutathione-S-transferase fusion protein; GST–Cip1) at a concentration that preferentially inhibits cyclin E–cdk2 rather than MPF(D'Angiolella et al. 2001). The inhibitor overcame checkpoint-dependent arrest restoring degradation of cyclin B and inactivation of cdk1 and cdk2 (Fig. 1C). Roscovitine, a different cdk inhibitor with equal potency for both cdk1 and cdk2, overcame arrest as well (Fig. 1D). MAPK activity, that plays a role in the checkpoint (Minshull et al. 1994), also dropped after cdk inhibition (Fig. 1E,F). GST–Cip1 (even at 2 μM; see below) or Roscovitine (2 μM) did not directly inhibit MAPK activity in kinase assays (data not shown). Exit from mitosis was confirmed by analysis of nuclear morphology (data not shown). Thus, both cdk1 and cdk2 activities are stabilized under checkpoint conditions, and inhibiting both cdks or just cdk2 overrides cell cycle arrest. Cdk2 may perform specific phosphorylations required by the checkpoint to block cell cycle progression. Alternatively, considering the effects of Roscovitine and that cdk2 is a potent activator of cdk1 in egg extracts (Guadagno and Newport 1996), cdk2 activity may be required to sustain cdk1 activity, and the latter activity may be directly involved in checkpoint control. The evidence we will show below indicates that cdk1 rather than cdk2 directly performs phosphorylations relevant to the checkpoint action.
Figure 1.
Cdk activities in checkpoint-arrested egg extracts. MPF (□; cyclin B–cdk1) and cdk2 (▪) kinase activities and autoradiographs of [35S]-labeled extracts proteins (the position of cyclin B is indicated) from portions of a cycling Xenopus egg extract during incubation at 23°C. (A) Control extract (+nocodazole). (B) Checkpoint-arrested extract (+demembranated sperm nuclei + nocodazole). Portions of the checkpoint-arrested extract received after 22 min of incubation: (C) GST–Cip1 (100 nM). (D) Roscovitine (2 μM; in DMSO). (E) MAPK activity in a checkpoint-arrested extract. (F) MAPK activity in a portion of the checkpoint-arrested extract that received GST–Cip1 (100 nM) after 22 min of incubation. The addition of GST or DMSO to checkpoint-arrested extracts did not reverse cell cycle arrest (data not shown).
Cdk activity is required for Mad2-dependent mitotic arrest
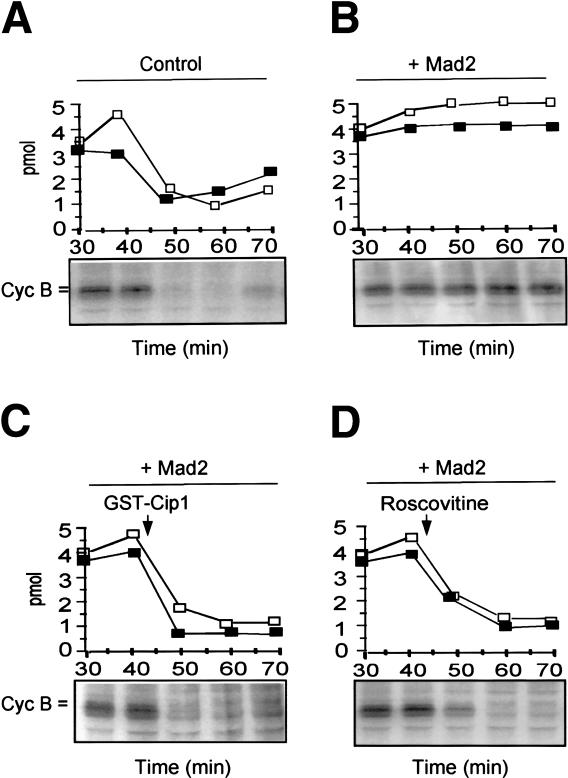
Unattached kinetochores activate the checkpoint protein Mad2 through complex mechanisms (Shah and Cleveland 2000). Activated Mad2 inhibits APC/CCdc20 by binding Cdc20 (Peters 2002). Checkpoint-dependent arrest in egg extracts depends on Mad2 (Chen et al. 1996). Mitotic cdk activity may affect Mad2 activation or the action of activated Mad2 on APC/CCdc20. The mechanisms that activate Mad2 are still not completely understood; however, it is known that an excess of recombinant Mad2 blocks APC/CCdc20 in egg extracts mimicking the effects of activated Mad2 (Li et al. 1997). We asked whether Mad2-induced mitotic arrest was sensitive to cdk inhibition. Stabilization of MPF activity and cyclin B was reached by adding recombinant Mad2 to a cycling extract (Fig. 2A,B). Cdk2 activity was also stabilized under these conditions (Fig. 2B). Furthermore, cell cycle arrest was rapidly overcome by addition of either p21/Cip1/Waf1 or Roscovitine (Fig. 2C,D). At higher Mad2 concentrations, however, mitotic arrest becomes resistant to cdk inhibitors (data not shown). These results indicate that mitotic cdk activity helps Mad2 to inhibit APC/CCdc20, although a role for cdk in Mad2 activation is not ruled out.
Figure 2.
Requirement for cdk activity in Mad2-induced mitotic arrest. MPF(□) and cdk2 (▪) kinase activities and autoradiographs of [35S]-labeled extracts proteins (the position of cyclin B is indicated) from portions of a cycling Xenopus egg extract during incubation at 23°C. (A) Control extract, buffer added prior to incubation. (B) Recombinant human Mad2 (80 μg/μL of extract; + Mad2) added prior to incubation. GST–Cip1 (C) or Roscovitine (D) were added to samples of the Mad2-treated extract portion after 40 min incubation. GST or DMSO did not reverse Mad2-dependent cell cycle arrest (data not shown).
Cdk activity affects the interactions of Cdc20 with Mad2 and APC/C in HeLa cells
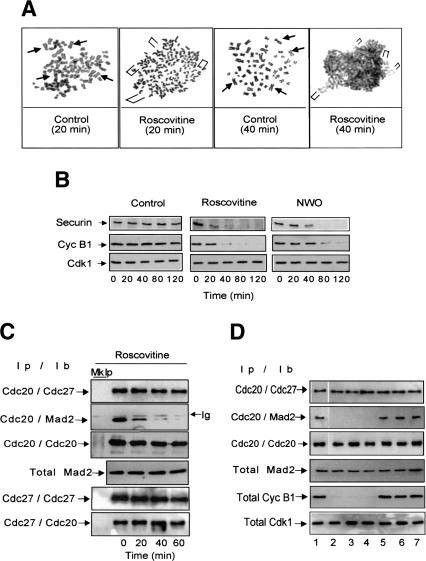
It has been shown that cdk inhibitors overcome spindle checkpoint-dependent arrest in somatic mammalian cells and in yeast. However, this has been interpreted as due to inappropriate APC/CCdh1 activation, otherwise inhibited by cdk activity (Zur and Brandeis 2001; Peters 2002). In the light of our observations in a system that depends only on APCCdc20 for degradation (Lorca et al. 1998), we reconsidered this issue asking whether sustained cdk activity is, instead, required to keep APC/ CCdc20 inhibited in checkpoint-arrested somatic human cells. We treated HeLa cells with nocodazole for 14 h to activate the checkpoint. Checkpoint-arrested cells were collected and either Roscovitine (dissolved in dymethylsulfoxide; DMSO) or DMSO, as control, was added in the continuous presence of nocodazole. Shortly after Roscovitine addition, sister chromatids lost cohesion and then started to decondense (Fig. 3A); securin was rapidly degraded followed by bulk cyclin B1 degradation (Fig. 3B). No significant changes were detected in chromosome morphology and protein stability after DMSO addition (Fig. 3A,B, control). The temporal degradation order of securin and cyclin B1 was similar to that observed in cells recovering from checkpoint arrest after nocodazole wash out (Fig. 3B, NWO).
Figure 3.
Cdk inhibition overrides checkpoint-dependent arrest in HeLa cells. Checkpoint-arrested HeLa cells were collected 14 h after nocodazole addition. DMSO, as control, or Roscovitine were then added in the continuous presence of nocodazole and samples taken at the indicated time points. (A) Chromosomes were stained with Giemsa. Arrowheads (Control) or brackets (Roscovitine) indicate maintenance or loss of sister chromatid cohesion. (B) Securin and cyclin B1 were visualized by immunoblot from total cell lysates; securin and cyclin B1 were also visualized from cells taken after nocodazole washout (NWO). In A, sister chromatid cohesion was lost in about 80% of cells 20 min after Roscovitine addition. (C) Cdc20 or Cdc27 were immunoprecipitated from samples taken at the indicated time points from Roscovitine addition (Ip) and associated Cdc27, Cdc20, and Mad2 were detected by immunoblot (Ib); the total amount of Mad2 is also shown. (Mk Ip) Mock precipitations; (Ig) immunoglobulin. (D) Checkpoint-arrested HeLa cells were collected 14 h after nocodazole addition and split into seven dishes. Then, DMSO (lane 1), Alsterpaullone (800 nM; lane 2), Indirubin 3′ monoxime (6 μM; lane 3), Purvalanol A (600 nM; lane 4), UO 126 (30 μM; lane 5), SB 203589 (30 μM; lane 6), and H89 (30 μM; lane 7), were individually added in the continuous presence of nocodazole, and cells harvested after additional 40 min incubation. Cdc20 was immunoprecipitated (Ip) and associated Cdc27 and Mad2 were detected by immunoblot (Ib), the total amount of Mad2, cyclin B1, and cdk1 is also shown. UO 126, SB 203589, and H89 were also tested in a range of concentration from 30 nM to 50 μM giving similar results (not shown).
When cells are released from checkpoint arrest by nocodazole wash out, activation of APC/CCdc20 requires Mad2 dissociation from Cdc20 (Fang et al. 1998). To know whether cdk inhibition relieved Mad2-dependent block of APC/CCdc20, we performed a series of experiments involving immunoprecipitation followed by immunoblotting to determine the interactions of Cdc27, an APC/C subunit, Cdc20, and Mad2 in checkpoint-arrested cells after Roscovitine addition. We found that the amount of Mad2 bound to Cdc20 was drastically reduced 20 min after Roscovitine addition, whereas Cdc20 binding to Cdc27 was maintained (Fig. 3C). Cdc20 progressively dissociated from Cdc27 presumably because of APC/C dephosphorylation (Fang et al. 1998; Kramer et al. 2000; Peters 2002). No significant changes were detected after 90 min from DMSO addition in control checkpoint-arrested cells (data not shown). Thus, cdk inhibition induces dissociation of Mad2 from Cdc20. Similar data were obtained using the nontransformed human cell line HUV-EC-C (data not shown). We also tested Alsterpaullone, Purvalanol A, and Indirubin 3′ monoxime—other known cell permeable inhibitors of cdk1—as well as UO 126, SB 203589, and H89, potent cell permeable inhibitor of other kinases like MEK1, p38, and PKA (Davies et al. 2000). Within 40 min of addition to checkpoint-arrested HeLa cells, all the cdk1 inhibitors reversed cell cycle arrest and induced dissociation of Mad2 from Cdc20 while the other inhibitors did not induce any of these events (Fig. 3D). Thus, although the MEKMAPK, p38 and PKA pathways have been implicated in the spindle checkpoint of mammalian cells (Peters 2002), their role appears more indirect than that of cdk1 for checkpoint control.
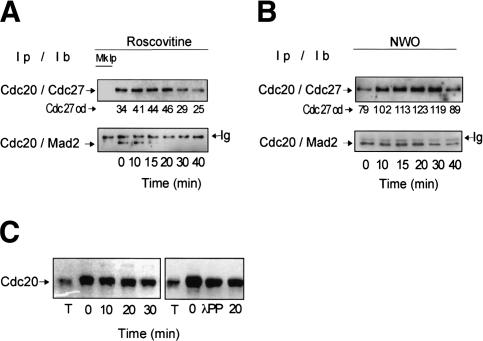
We also found that the interaction of Cdc20 with Cdc27 increased early after cdk inhibition. This was better observed at very short time points after Roscovitine addition (Fig. 4A; similar results were also obtained with Indirubin 3′ monxime or Purvalanol A; data not shown). The transient increase in Cdc20–Cdc27 interaction could also be observed in cells released from checkpoint arrest shortly after nocodazole washout (Fig. 4B). These findings are consistent with the observation that cdk phosphorylation of Cdc20 inhibits binding to Cdc27 in vitro (Yudkovsky et al. 2000). We conclude that cdk activity restrains APC/CCdc20 activation until proper assembly of the mitotic spindle in somatic human cells by stabilizing Cdc20–Mad2 interaction and reducing Cdc20 binding to APC/C.
Figure 4.
Cdk1 inhibition affects Cdc20 phosphorylation and binding to Cdc27 and Mad2 in Hela cells. Cdc20 was immunoprecipitated (Ip) and associated Cdc27 or Mad2 were detected by immunoblot (Ib) from checkpoint-arrested cells taken at the indicated time points from Roscovitine addition (A) and nocodazole washout (B). Optical density values of Cdc27 bound to Cdc20 are indicated (Cdc27 od; arbitrary units). (Mk Ip) Mock precipitations. (C, left) Immunoblot of Cdc20 from thymidine-arrested (T) and from nocodazole-arrested HeLa cells taken at the indicated time points after Roscovitine addition. (Right) Immunoblot of Cdc20 from thymidine-arrested cells (T), from nocodazole-arrested cells nontreated (0) and treated (λPP) in vitro with λ protein phosphatase and from nocodazole-arrested cells after 20 min from Roscovitine addition (20).
Cdc20 binding to Cdc27 and Mad2 is affected by cdk1 phosphorylation
A possible target for cdk action in regulating Cdc20 interaction with APC/C and Mad2 could be Cdc20 itself. Cdc20 contains multiple cdk phosphorylation sites and is phosphorylated during mitosis and under checkpoint conditions (Kramer et al. 2000; Yudkovsky et al. 2000; Peters 2002). Mutation of several of these sites substantially reduces Cdc20 phosphorylation by mitotic egg extracts and by cdk1 (Kramer et al. 2000; Yudkovsky et al. 2000). In addition, it has been shown by in vitro studies that phosphorylated Cdc20 is a poorer stimulator of APC/C activity than non- or hypophosphorylated Cdc20 (Kramer et al. 2000) and that cdk-dependent phosphorylation of Cdc20 inhibits binding to Cdc27 (Yudkovsky et al. 2000). Thus, under checkpoint conditions, cdk1-dependent phosphorylation of Cdc20 may affect the ability of Cdc20 to bind APC/C and Mad2. Phosphorylated Cdc20 has retarded mobility on SDS-PAGE (Fang et al. 1998; Kramer et al. 2000). To analyze the phosphorylation status of Cdc20 in checkpoint-arrested cells after cdk inhibition, we separated HeLa proteins in longer SDS-PAGE runs to better visualize changes in Cdc20 mobility. Cdc20 mobility increased after Roscovitine addition to checkpoint-arrested cells, suggesting that it becomes dephosphorylated (Fig. 4C; similar results were also obtained with Indirubin 3′ monxime or Purvalanol A; data not shown). Cdc20 isolated from checkpoint-arrested cells also showed increased mobility after treatment with λ protein phosphatase, indicating that the increased mobility can be ascribed to dephosphorylation (Fig. 4C). The timing of Cdc20 dephosphorylation is compatible with the hypothesis that dephosphorylation of Cdc20 increases binding to APC/C while it decreases it to Mad2.
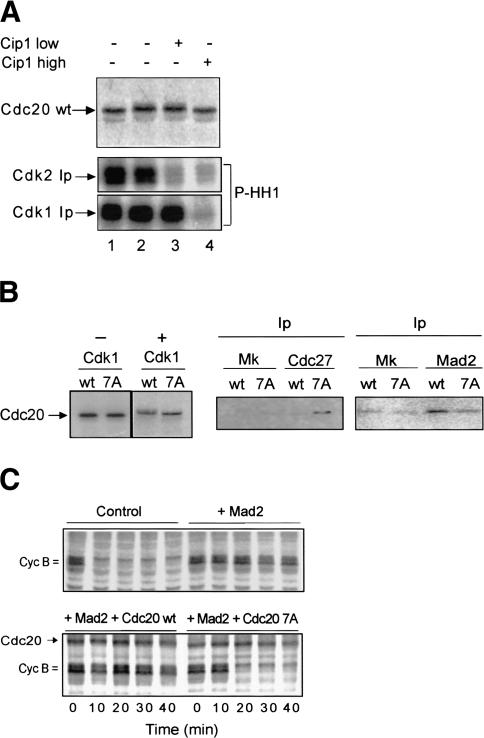
We used egg extracts to study in more detail the relevance of Cdc20 phosphorylation for the interaction with APC/C and Mad2. We, first, asked whether Cdc20 phosphorylation depended on cdk1 or cdk2 activity in egg extracts. To this end we used cytostatic factor-arrested (CSF) M-phase extracts. In these extracts, cdk1 activity is sustained by redundant mechanisms so that cdk2 can be inhibited leaving cdk1 activity unaffected (Tunquist et al 2002). After 30 min incubation in CSF-arrested extracts, [35S]-labeled Cdc20 wild type underwent phosphorylation as shown by the previously described (Kramer et al. 2000) mobility shift on SDS-PAGE (Fig. 5A, cf. lanes 1 and 2). Low concentration of p21/ Cip1/Waf1, added from time 0 of incubation, selectively inhibited cdk2 but did not prevent the Cdc20 wild-type gel mobility shift (Fig. 5A, lane 3). On the contrary, high concentrations of p21/Cip1/Waf1 inhibited also cdk1 and prevented the Cdc20 wild-type gel mobility shift (Fig. 5A, lanes 4,5). These results indicate that cdk1 rather than cdk2 is responsible for Cdc20 phosphorylation.
Figure 5.
Cdk1-dependent phosphorylation of Cdc20 is required for Mad2-dependent arrest. (A) [35S]-labeled Cdc20 wild-type (wt) was produced in reticulocyte lysates and incubated with portions of a CSF-arrested egg extract in the presence of cycloheximide. Samples were taken at time 0 (lane 1), after 30 min incubation without further additions (lane 2), after 30 min incubation in the presence of 100 nM GST–Cip1 added from time 0 (lane 3; Cip1 low), and after 30 min incubation in the presence of 2 μM GST–Cip1 added from time 0 (lane 4; Cip1 high). Autoradiographs show [35S]-labeled Cdc20 in the extract samples (top), histone H 1 phosphorylated (P-HH1) by cdk2 (middle), or cdk1 (bottom) immunoprecipitated (Ip) from extract samples 1, 2, 3, and 4. (B) [35S]-labeled Cdc20 wild type (wt) and seven-phosphorylation-sites mutant (7A) were produced in reticulocyte lysates (-Cdk1) and incubated with active cdk1 (+Cdk1). Phosphorylated wild-type and 7A Cdc20 were then incubated with portions of a CSF-arrested extract containing recombinant human Mad2 (80 μg/μL of extract) and cycloheximide for 30 min at 23°C. Cdc27 or Mad2 were immunoprecipitated (Ip) and the amount of bound wild-type (wt) and 7A Cdc20 detected by autoradiography. (Mk) Mock precipitations. (C) A CSF-arrested extract was labeled with [35S]-methionine and cysteine for 30 min, then cycloheximide and buffer (Control) or recombinant human Mad2 (+Mad2) were added and incubation prolonged for 30 min at 23°C. Subsequently, cdk1 phosphorylated Cdc20 wild type and 7A were added to Mad2 containing extract portions (1 reticulocyte lysate/40 extract) and incubation further prolonged for 5 min. CaCl2 was then added, and samples taken at the indicated time points. The addition of nonprogrammed reticulocyte lysate (up to 1/10 extract volume) did not affect Mad2-dependent arrest.
To know whether cdk1-dependent phosphorylation affected the ability of Cdc20 to interact with Cdc27 and Mad2 in egg extracts, we used [35S]-labeled Cdc20 wild type and a previously described (Yudkovsky et al. 2000) mutant Cdc20 version nonphosphorylatable at seven cdk phosphorylation sites (7A). Using this mutant, it has been shown that cdk1-phosphorylated Cdc20 binds poorly to Cdc27 in vitro (Yudkovsky et al. 2000). Cdc20 wild type and 7A were independently pretreated with active cdk1 (Fig. 5B, -Cdk1 and +Cdk1) and then incubated in CSF-arrested extracts with recombinant Mad2. Cdc27 or Mad2 were isolated 30 min later and the amount of bound Cdc20 wild type or 7A was determined. Higher binding of Cdc20 7A than wild type was observed to Cdc27, consistent with previous observations (Fig. 5B; Yudkovsky et al. 2000). Conversely, Mad2 preferentially bound Cdc20 wild type than 7A (Fig. 5B). Although cdk-dependent phosphorylation of Cdc20 in vivo may not be so extensive as in vitro, these data show that cdk-phosphorylated Cdc20 interacts more efficiently with Mad2 than Cdc27. This is consistent with the in vivo findings that cdk inhibition induces dissociation of Cdc20 from Mad2 and a transient increase of Cdc20 binding to Cdc27 (Figs. 3C, 5B).
We next tested whether Cdc20 7A, resistant to cdk1 phosphorylation, could overcome Mad2-dependent mitotic arrest. A CSF-arrested extract was preincubated for 30 min with excess of Mad2. Then, similar amounts of Cdc20 wild type and 7A, prephosphorylated by cdk1, were added to the Mad2-containing extract. Incubation was prolonged for 5 min and then CaCl2 was added to inactivate CSF. In the control extract cyclins were rapidly degraded after CaCl2 addition (Fig. 5C). In the extract treated with excess of Mad2, cyclins remained stable for at least 40 min after CaCl2 addition. Cyclins remained stable as well in the extract containing Mad2 and Cdc20 wild type. Cyclin degradation was, instead, restored in the extract containing Mad2 and Cdc20 7A (by 20 min; Fig. 5C). Thus, Mad2-dependent arrest was maintained in the presence of the cdk1-phosphorylated wild-type Cdc20 but not of the phosphorylation-resistant mutant Cdc20 version. Similar results were obtained when the checkpoint was activated by nuclei and nocodazole (data not shown).
Our observations, from Xenopus egg extracts and human somatic cells, indicate a crucial role for cdk1 activity in the regulation of the spindle checkpoint. Cdk1 activity appears required to inhibit APC/CCdc20 activation by stabilizing Cdc20–Mad2 interaction and reducing Cdc20 binding to APC/C via direct phosphorylation of Cdc20.
In Xenopus egg extracts and embryos, cyclin E–cdk2 activity is relevant for many aspects of the M phase, and we find it also involved in checkpoint control. It is possible that, under checkpoint conditions, cyclin E–cdk2 is required to sustain cdk1 activity (Guadagno and Newport 1996), and that this is ultimately responsible to inhibit APC/CCdc20 activation (Fig. 5A). It is presently unclear why cyclin E–cdk2 plays a role in mitotic events in this system, while it does not appear to be so relevant for mitosis in somatic cells (Tunquist and Maller 2003). Many reports also indicate that MAPK participates in checkpoint regulation in egg extracts (Tunquist et al 2002). MAPK may participate directly in checkpoint regulation, as suggested by recent evidence (Chung and Chen 2003), and also indirectly, like cdk2, by sustaining cdk1 activity (Tunquist and Maller 2003).
In mammalian cells, cdk2 may not have a crucial role in spindle checkpoint-dependent arrest because the abundance of its partners, cyclin A and E, is very low under checkpoint conditions (Peters 2002), and the relevance of the MAPK pathway in mitosis and spindle checkpoint is not yet completely understood (Roberts et al. 2002). Some human cancers, however, show deregulated cyclin E expression and cyclin E–cdk2 activity is present at high levels also in mitosis (Gray-Bablin et al. 1996). Our observations in the embryonic system may provide a framework to explain some aspects of the oncogenic potential of deregulated cyclin E expression and why this causes chromosomal instability in somatic mammalian cells (Spruck et al. 1999).
During mitosis, cdk1 may perform positive phosphorylations of APC/C, a prerequisite for its activity (Peters 2002), but at the same time, by phosphorylating Cdc20, it lessens interaction between APC/C and Cdc20 and provides a precondition for inhibitory checkpoint proteins, activated at unattached kinetochores like Mad2, to interact with Cdc20. Thus, upon completion of mitotic spindle assembly, activated checkpoint proteins are no longer produced and active APC/Cdc20 can begin to form. An alternative hypothesis could be that the checkpoint mechanism acts also by sustaining the pathways that control and maintain cdk activation (Grieco et al. 1996; D'Angiolella et al. 2001). Thus, upon completion of mitotic spindle assembly, an early and transient proteolysis-independent drop in cdk1 activity may help rapid APC/Cdc20 reactivation by increasing the Cdc20–APC/C interaction and inducing the dissociation of activated checkpoint proteins from Cdc20.
Materials and methods
Egg extracts and cell culture
Xenopus egg extracts were prepared and spindle checkpoint activation was obtained essentially as described (Grieco et al. 1994; Minshull et al. 1994). Extracts proteins were labeled by adding [35S]-methionine (400 μCi/mL; Amersham) to extracts at time 0 of incubation. MPF and cdk2 kinase activities were measured as previously described (D'Angiolella et al. 2001). MAPK activity was measured as myelin basic protein (MBP) kinase activity in anti-Xen-MAPK (p42) immunoprecipitates (IPs; phosphorylated MBP was quantified by densitometry). HeLa were grown in DMEM (GIBCO) supplemented with 10% fetal calf serum, glutamine, and penicillin/streptomycin. To activate the checkpoint, cells were first treated for 18 h with 2 mM thymidine, then released for 3 h into fresh medium before nocodazole (400 ng/mL; Calbiochem) addition. Fourteen hours later, mitotic cells were recovered by shake-off and allowed to rest for 30 min in new dishes. Then, either DMSO or Rosacovitine (30 μM; Calbiochem) in DMSO were added. For nocodazole wash-out experiments, mitotic cells were recovered by shake-off and washed once with prewarmed phosphase-buffered saline (PBS) before incubation in prewarmed fresh medium; time 0 corresponds to cells that were washed once in ice-cold PBS and immediately lysed. To visualize chromosomes, cells were first incubated with hypotonic medium (1:4 with dH2O), fixed with methanol, and stained with Giemsa (Sigma). For cell extracts, cell pellets were lysed with 5 volumes of 15 mM HEPES at pH 7.4, 400 mM NaCl, 0.1% NP40, and protease inhibitors. Lysates were incubated 30 min on ice and then spun two times for 20 min at 13,000 rpm in a refrigerated microfuge. λ protein phosphatase treatment (Calbiochem) was performed on Cdc20 immunoprecipitated from nocodazole arrested HeLa cell as previously described (Kramer et al. 2000).
Antibodies and immunoprecipitations
Anti-Xenopus MAPK, anti-Xenopus cdk2, and antihuman Mad2 antibodies were a generous gift of Dr. J. Maller (Department of Pharmacology, University of Colorado, Denver) and Dr. R. Benezra (Memorial Sloan-Kettering Cancer Center, New York). All other antibodies were purchased from Santa Cruz Biotechnology. IPs from egg extracts were performed as follows: 5–15 μL of extracts samples were diluted in 250 μL ice-cold buffer containing 15 mM HEPES at pH 7.4, 150 mM NaCl, 0.1% NP40, and protease inhibitors. Samples were incubated with 2 μg antibody for 2 h on ice, mixed with 20 μL of 50% protein A + G-sepharose suspension, and further incubated with rotation at 4°C for 2 h. Bead pellets were washed two times with low (150 mM NaCl) and three times with high (400 mM NaCl) salt buffer and a final wash in kinase reaction buffer (Grieco et al. 1994). IPs from HeLa cells were performed similarly starting from 400-μL extract using 4 μg antibody and 30 μL of 50% protein A + G-sepharose suspension. Mock precipitations (MK) were performed with nonimmune Ig from time 0 samples. Primary antibodies on immunoblots were revealed by proxidase-conjugated protein A and protein G (Bio-Rad) using an ECL kit (Amersham) according to the manufacturer's instructions.
Recombinant proteins
Plasmids to express Cdc20 wild type and 7A were a generous gift of Dr. A. Hershko (Technion-Israel Institute of Technology, Haifa, Israel). Proteins were in vitro transcribed and translated in the presence of [35S]-labeled methionine and cysteine (promix; Amersham) using a T7–TNT coupled system (Promega). Plasmid to express histidine-tagged-human Mad2 was a generous gift of R. Benezra. Bacterially expressed 6X-histidine-Mad2 was prepared by Ni–NTA–agarose (Qiagen) chromatography essentially as described by the manufacturer. After binding, the Ni–N-TA–agarose column was washed with 100 volumes of 5 mM imidazole, 15 mM HEPES at pH 7.4, 150 mM NaCl, 0.1% NP40, and protease inhibitors. Protein was eluted with 250 mM imidazole, 20 mM HEPES, 50 mM KCl. Imidazole was removed by concentration-dilution cycles using centricon 10 (Amicon). Active cdk1 for in vitro phosphorylation reactions was isolated by IP from CSF-arrested extracts. Phosphorylation reactions with in vitro transcribed-translated Cdc20 proteins were performed as described (Yudkovsky et al. 2002). For incubation with egg extracts, 1 volume of reticulocyte lysates was incubated with 20 volumes of egg extract. Endogenous reticulocyte lysates Cdc27 was immunodepleted after in in vitro translation and prior to phosphorylation.
Acknowledgments
We thank J. Maller, A. Hershko, and R. Benezra for generously providing reagents; P. Malatesta and D. Tramontano for help with cytogenetic experiments; and Rita Cerillo for technical advice. This work was supported by a NUSUG grant of the Associazione Italiana Ricerca Cancro (AIRC) to D.G..
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.267603.
References
- Chen R.H., Waters, J.C., Salmon, E.D., and Murray, A.W. 1996. Association of spindle assembly checkpoint component xmad2 with unattached kinetochores. Science 274: 242-246. [DOI] [PubMed] [Google Scholar]
- Chung E. and Chen, R.H. 2003. Phosphorylation of Cdc20 is required for its inhibition by the spindle checkpoint. Nat. Cell Biol. Jul 13: (Epub ahead of print). [DOI] [PubMed]
- D'Angiolella V., Costanzo, V., Gottesman, M.E., Avvedimento, E.V., Gautier, J., and Grieco, D. 2001. Role for Cyclin-dependent kinase 2 in mitosis exit. Curr. Biol. 11: 1221-1226. [DOI] [PubMed] [Google Scholar]
- Davies S.P, Reddy, H., Caivano, M., and Cohen, P. 2000. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351: 95-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulic V., Lees, E., and Reed, S.I. 1992. Association of human cyclin E with a periodic G1–S phase protein kinase. Science 257: 1958-1961. [DOI] [PubMed] [Google Scholar]
- Fang G., Hongtao, Y., and Kirschner, M.W. 1998. The checkpoint protein Mad2 and the mitotic regulator CDC20 form a ternary complex with the anaphase-promoting complex to control anaphase initiation. Genes & Dev. 12: 1871-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray-Bablin J., Zalvide, J., Fox, M.P, Knickerbocker, C.J., DeCaprio, J.A., and Keyomarsi, K. 1996. Cyclin E, a redundant cyclin in breast cancer. Proc. Natl. Acad. Sci. 93: 15215-15220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco D., Avvedimento, V.E., and Gottesman, M.E. 1994. A role for cAMP-dependent kinase in early embryonic divisions. Proc. Natl. Acad. Sci. 91: 9896-9900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco D., Porcellini, A., Avvedimento, V.E., and Gottesman, M.E. 1996. Requirement for cAMP-PKA pathway activation by M phase promoting factor in the transition from mitosis to interphase. Science 271: 1718-1722. [DOI] [PubMed] [Google Scholar]
- Guadagno T.M. and Newport, J.W. 1996. Cdk2 kinase is required for entry into mitosis as a positive regulator of Cdc2–cyclin B kinase activity. Cell 84: 73-82. [DOI] [PubMed] [Google Scholar]
- Kramer E.R., Sheuringer, N., Podtelejnikov, A.V., Mann, M., and Peters, J. M. 2000. Mitotic regulation of the APC activator proteins CDC20 and CDH1. Mol. Biol. Cell 11: 1555-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Gorbea, C., Mahaffey, D., Rechsteiner, M., and Benezra, R. 1997. MAD2 associates with the cyclosome/anaphase-promoting complex and inhibits its activity. Proc. Natl. Acad. Sci. 94: 12431-12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorca T., Castro, A., Martinez, A.M., Vigneron, S., Mori, N., Sigrist, S., Lehner, C., Doree, M., and Labbe, J.C. 1998. Fizzy is required for activation of the APC/cyclosome in Xenopus egg extracts. EMBO J. 17: 3565-3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshull J., Sun, H., Tonks, N.K., and Murray, A.W. 1994. A MAPK-dependent spindle assembly checkpoint in Xenopus egg extracts. Cell 79: 475-486. [DOI] [PubMed] [Google Scholar]
- Peters J.M. 2002. The anaphase-promoting complex: Proteolysis in mitosis and beyond. Mol. Cell 9: 931-943. [DOI] [PubMed] [Google Scholar]
- Roberts E.C., Shapiro, P.S., Stines Nahreini, T., Pages, P., Pouyssegur, J., and Ahn, N.G. 2002. Distinct cell cycle timing requirements for extracellular signal-regulated kinase and phosphoinositide 3-kinase signalling pathways in somatic cell mitosis. Mol. Cell. Biol. 22: 7226-7241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah J.V. and Cleveland, D.W. 2000. Waiting for anaphase: mad2 and the spindle assembly checkpoint. Cell 103: 997-1000. [DOI] [PubMed] [Google Scholar]
- Spruck C.H., Wong, K.A., and Reed, S.I. 1999. Deregulated cyclin E induces chromosome instability. Nature 401: 297-300. [DOI] [PubMed] [Google Scholar]
- Tunquist B.J. and Maller, J.L. 2003. Under arrest: Cytostatic factor (CSF)-mediated metaphase arrest in vertebrate eggs. Genes & Dev. 17: 683-710. [DOI] [PubMed] [Google Scholar]
- Tunquist B.J., Schwab, M.S., Chen, L.G., and Maller, J.L. 2002. The spindle checkpoint kinase bub1 and cyclin e/cdk2 both contribute to the establishment of meiotic metaphase arrest by cytostatic factor. Curr. Biol. 12: 1027-1033. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S., Decottignies, A., and Nurse, P. 2003. Function of Cdc2p-dependent Bub1p phosphorylation and Bub1p kinase activity in the mitotic and meiotic spindle checkpoint. EMBO J. 22: 1075-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkovsky Y., Shteinberg, M., Listovsky, T., Brandeis, M., and Hershko, A. 2000. Phosphorylation of Cdc20/fizzy negatively regulates the mammalian cyclosome/APC in the mitotic checkpoint. Biochem. Biophys. Res. Commun. 271: 299-304. [DOI] [PubMed] [Google Scholar]
- Zur A. and Brandeis, M. 2001. Securin degradation is mediated by fzy and fzr, and is required for complete chromatid separation but not for cytokinesis. EMBO J. 20: 792-801. [DOI] [PMC free article] [PubMed] [Google Scholar]