Abstract
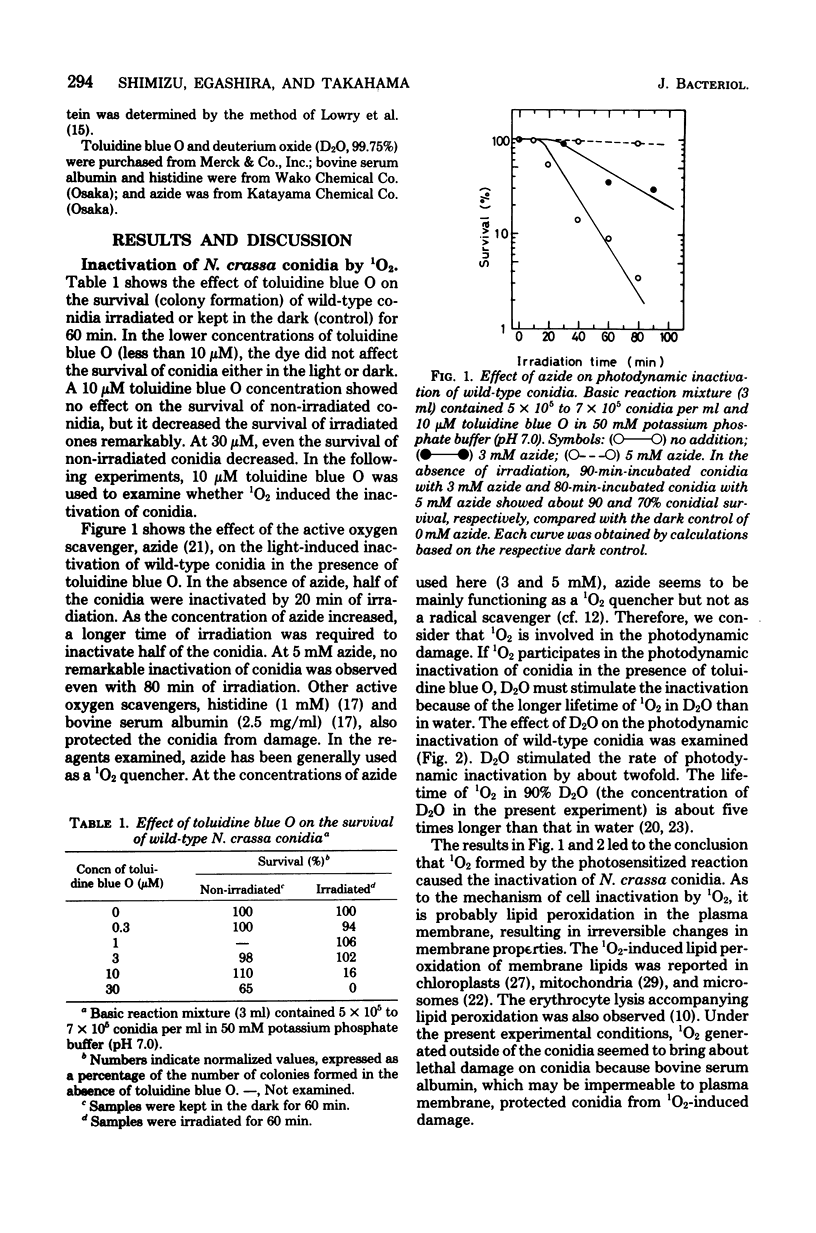
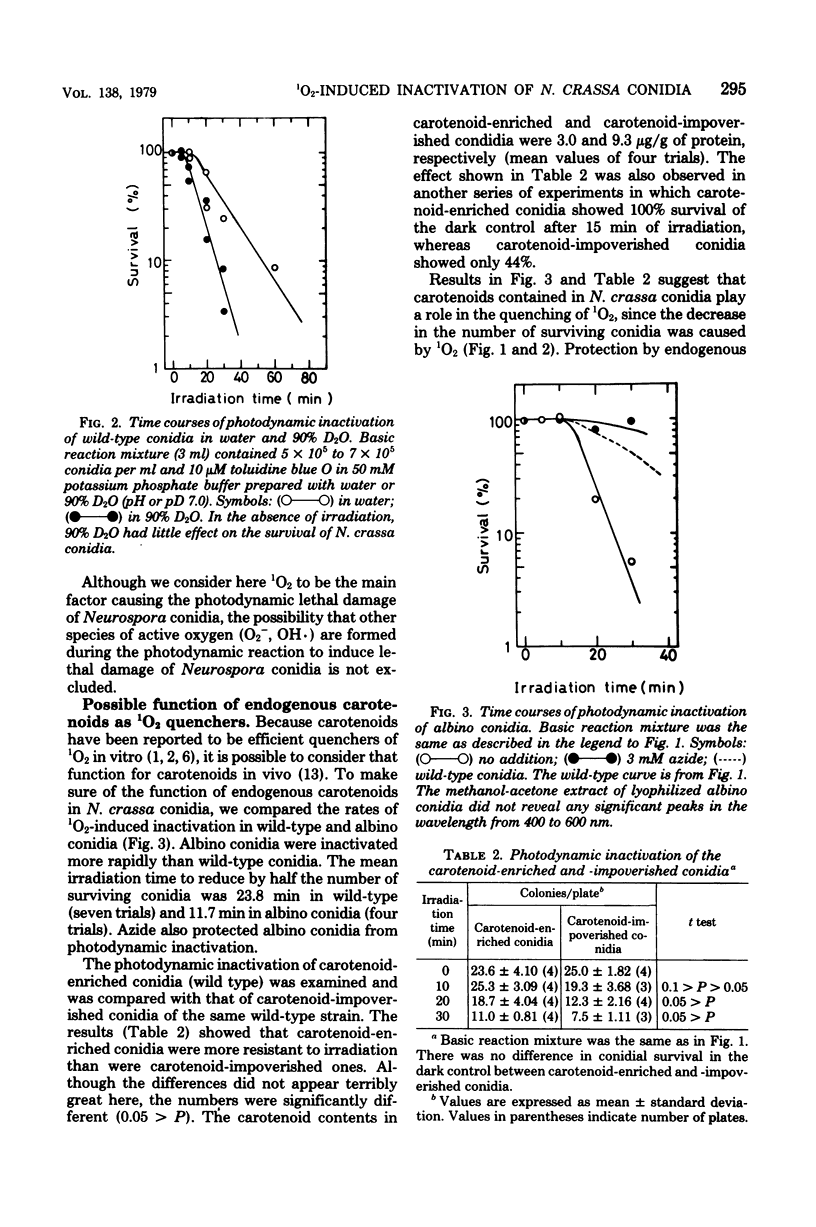
Photodynamic damage of Neurospora crassa conidia was studied in the presence of the photosensitizing dye, toluidine blue O. Conidia which germinated to form colonies decreased in number as irradiation time became longer. The photoinactivation of conidia was suppressed by azide, bovine serum albumin, and histidine, and was stimulated in deuterium oxide. Wild-type conidia were less sensitive to the irradiation than albino conidia. In the wild-type, carotenoid-enriched conidia were more resistant against the lethal damage than the conidia which contained small amounts of carotenoids. These results suggest that singlet molecular oxygen causes photodynamic lethal damage to N. crassa conidia and that singlet molecular oxygen is quenched by endogenous carotenoids.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. M., Krinsky N. I. Protective action of carotenoid pigments against photodynamic damage to liposomes. Photochem Photobiol. 1973 Nov;18(5):403–408. doi: 10.1111/j.1751-1097.1973.tb06440.x. [DOI] [PubMed] [Google Scholar]
- Anderson S. M., Krinsky N. I., Stone M. J., Clagett D. C. Effect of singlet oxygen quenchers on oxidative damage to liposomes initiated by photosensitization or by radiofrequency discharge. Photochem Photobiol. 1974 Jul;20(1):65–69. doi: 10.1111/j.1751-1097.1974.tb06549.x. [DOI] [PubMed] [Google Scholar]
- Blanc P. L., Tuveson R. W., Sargent M. L. Inactivation of carotenoid-producing and albino strains of Neurospora crassa by visible light, blacklight, and ultraviolet radiation. J Bacteriol. 1976 Feb;125(2):616–625. doi: 10.1128/jb.125.2.616-625.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn G. E., Tseng H. Y. Photodynamic inactivation of yeast sensitized by eosin Y. Photochem Photobiol. 1977 Nov;26(5):465–474. doi: 10.1111/j.1751-1097.1977.tb07516.x. [DOI] [PubMed] [Google Scholar]
- Foote C. S., Chang Y. C., Denny R. W. Chemistry of singlet oxygen. X. Carotenoid quenching parallels biological protection. J Am Chem Soc. 1970 Aug 26;92(17):5216–5218. doi: 10.1021/ja00720a036. [DOI] [PubMed] [Google Scholar]
- Harding R. W., Huang P. C., Mitchell H. K. Photochemical studies of the carotenoid biosynthetic pathway in Neurospora crassa. Arch Biochem Biophys. 1969 Feb;129(2):696–707. doi: 10.1016/0003-9861(69)90230-6. [DOI] [PubMed] [Google Scholar]
- Ito T. Toluidine blue: the mode of photodynamic action in yeast cells. Photochem Photobiol. 1977 Jan;25(1):47–53. doi: 10.1111/j.1751-1097.1977.tb07423.x. [DOI] [PubMed] [Google Scholar]
- Kellogg E. W., 3rd, Fridovich I. Liposome oxidation and erythrocyte lysis by enzymically generated superoxide and hydrogen peroxide. J Biol Chem. 1977 Oct 10;252(19):6721–6728. [PubMed] [Google Scholar]
- Kobayashi K., Ito T. Further in vivo studies on the participation of singlet oxygen in the photodynamic inactivation and induction of genetic changes in Saccharomyces cerevisiae. Photochem Photobiol. 1976 Jan;23(1):21–28. doi: 10.1111/j.1751-1097.1976.tb06765.x. [DOI] [PubMed] [Google Scholar]
- Krinsky N. I. Singlet excited oxygen as a mediator of the antibacterial action of leukocytes. Science. 1974 Oct 25;186(4161):363–365. doi: 10.1126/science.186.4161.363. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matheson I. B., Etheridge R. D., Kratowich N. R., Lee J. The quenching of singlet oxygen by amino acids and proteins. Photochem Photobiol. 1975 Mar;21(3):165–171. doi: 10.1111/j.1751-1097.1975.tb06647.x. [DOI] [PubMed] [Google Scholar]
- Mathews-Roth M. M., Krinsky N. I. Studies on the protective function of the carotenoid pigments of Sarcina lutea. Photochem Photobiol. 1970 Jun;11(6):419–428. doi: 10.1111/j.1751-1097.1970.tb06014.x. [DOI] [PubMed] [Google Scholar]
- Mathews-Roth M. M., Wilson T., Fujimori E., Krinsky N. I. Carotenoid chromophore length and protection against photosensitization. Photochem Photobiol. 1974 Mar;19(3):217–222. doi: 10.1111/j.1751-1097.1974.tb06501.x. [DOI] [PubMed] [Google Scholar]
- Nilsson R., Merkel P. B., Kearns D. R. Kinetic properties of the triplet states of methylene blue and other photosensitizing dyes. Photochem Photobiol. 1972 Aug;16(2):109–116. doi: 10.1111/j.1751-1097.1972.tb07342.x. [DOI] [PubMed] [Google Scholar]
- Pederson T. C., Aust S. D. The role of superoxide and singlet oxygen in lipid peroxidation promoted by xanthine oxidase. Biochem Biophys Res Commun. 1973 Jun 8;52(3):1071–1078. doi: 10.1016/0006-291x(73)91047-4. [DOI] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Formation of singlet oxygen by the myeloperoxidase-mediated antimicrobial system. J Biol Chem. 1977 Jul 25;252(14):4803–4810. [PubMed] [Google Scholar]
- Schmidt H., Rosenkranz P. On the mechanism of the acridine orange sensitized photodynamic inactivation of lysozyme. I. Basic kinetics. Z Naturforsch C. 1976 Jan-Feb;31(1-2):29–39. doi: 10.1515/znc-1976-1-209. [DOI] [PubMed] [Google Scholar]
- Singh H., Vadasz J. A. Singlet oxygen quenchers and the photodynamic inactivation of E. coli ribosomes by methylene blue. Biochem Biophys Res Commun. 1976 May 23;76(2):391–397. doi: 10.1016/0006-291x(77)90737-9. [DOI] [PubMed] [Google Scholar]
- Tatum E. L., Barratt R. W., Cutter V. M., Jr Chemical Induction of Colonial Paramorphs in Neurospora and Syncephalastrum. Science. 1949 May 20;109(2838):509–511. doi: 10.1126/science.109.2838.509. [DOI] [PubMed] [Google Scholar]
- Zimmermann R., Flohé L., Weser U., Hartmann H. J. Inhibition of lipid peroxidation in isolated inner membrane of rat liver mitochondria by superoxide dismutase. FEBS Lett. 1973 Jan 15;29(2):117–120. doi: 10.1016/0014-5793(73)80539-3. [DOI] [PubMed] [Google Scholar]



