Abstract
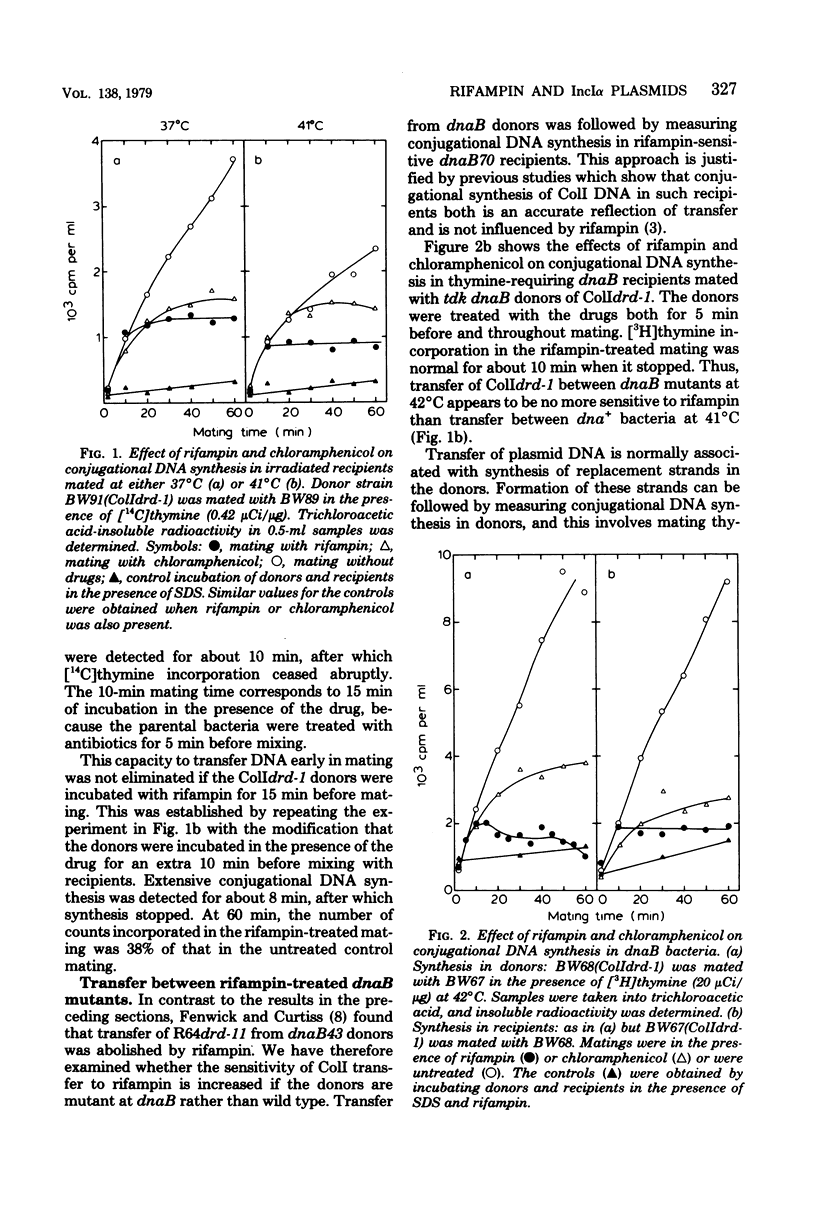
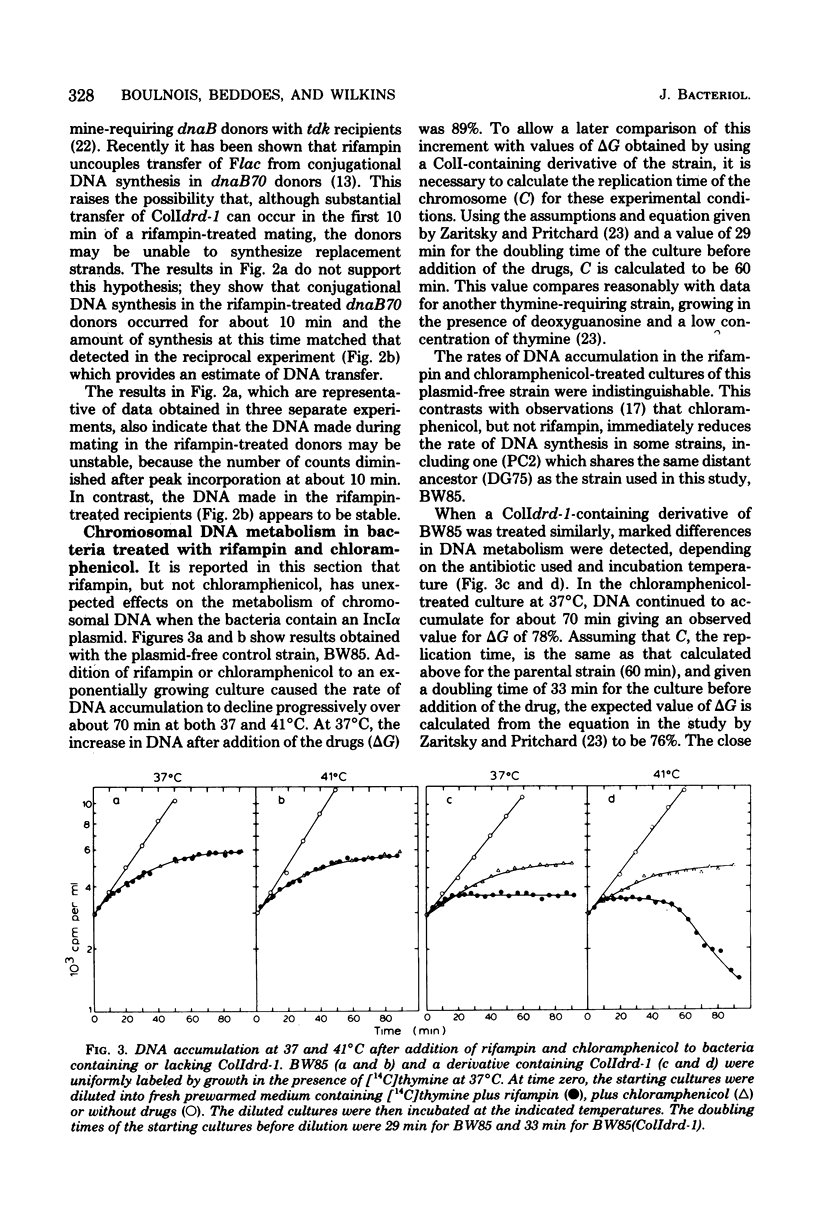
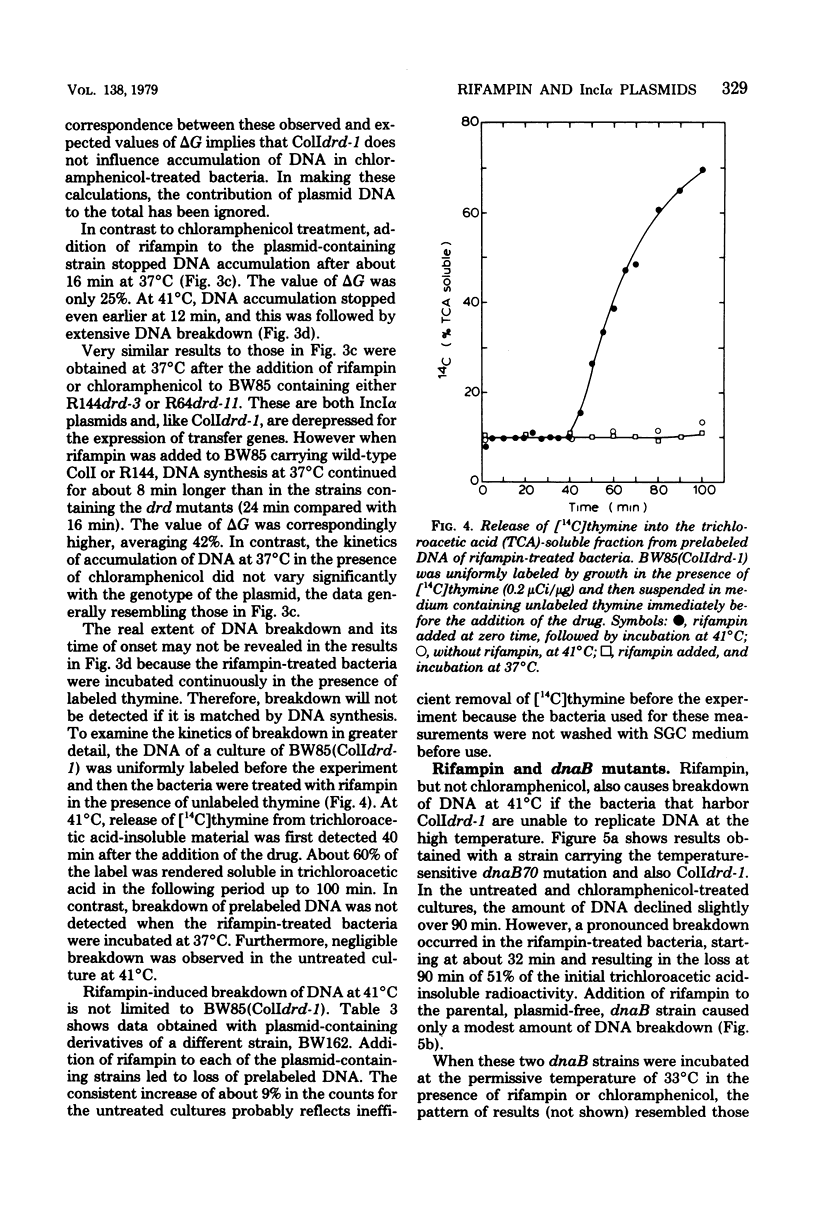
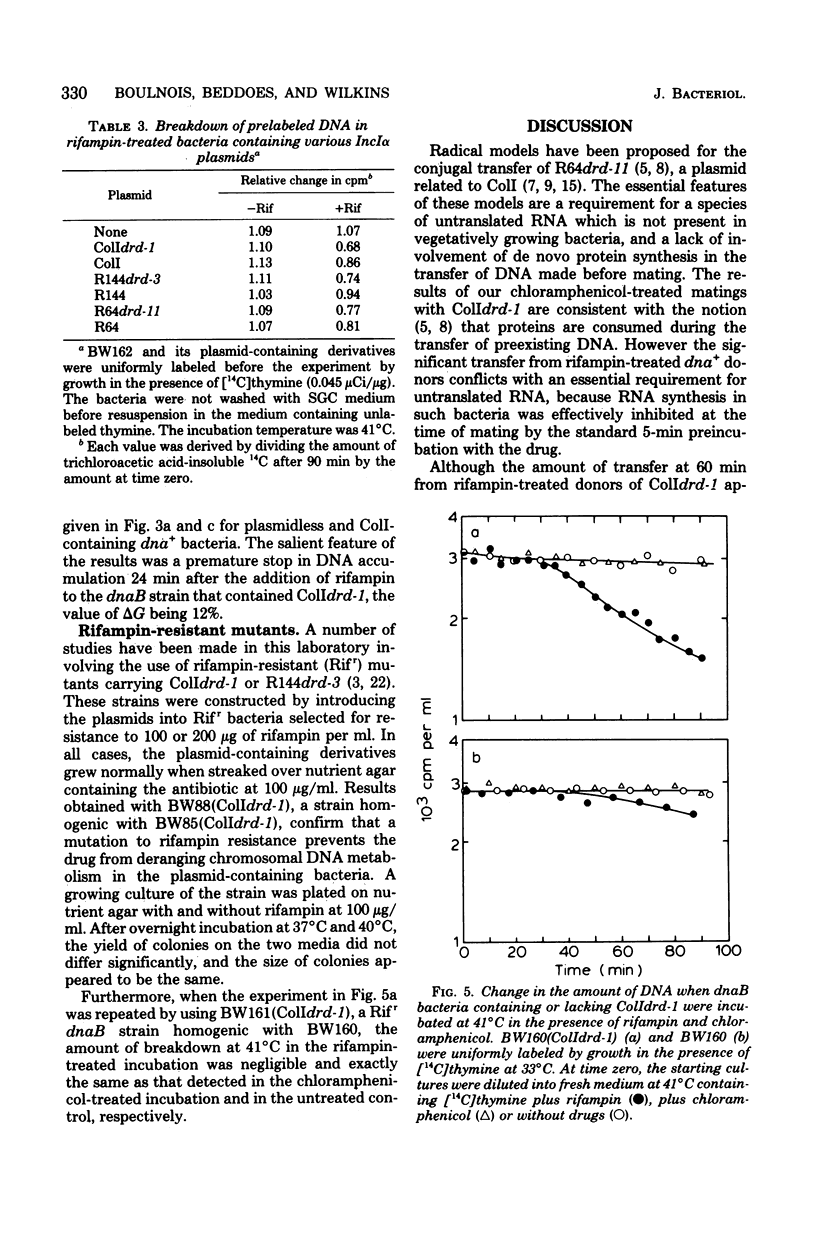
The effects of rifampin and chloramphenicol on the transfer of ColIdrd-1 have been examined to determined whether transfer requires the synthesis of an untranslated species of ribonucleic acid (RNA), as proposed in models for the transfer of another IncIalpha plasmid, R64drd-11. When RNA synthesis was inhibited throughout mating by rifampin, ColI transfer between dna+ bacteria occurred at the normal rate for about 10 min and then stopped abruptly. Conjugational deoxyribonucleic acid (DNA) synthesis in dnaB mutants indicates that plasmid DNA was made in the rifampin-treated donors to replace the transferred material but the DNA tended to be unstable. In the presence of chloramphenicol, transfer of ColI gradually diminished over a longer period. Rifampin, but not chloramphenicol, was found to have unpredicted effects on chromosomal DNA metabolism in unmated dna+ and dnaB bacteria when they harbor any of three IncIalpha plasmids (ColIdrd-1, R144drd-3, and R64drd-11). Replication of the bacterial chromosome in such cells stopped abruptly about 15 min after the addition of rifampin, and at 41 degrees C, but not 37 degrees C, this was followed by extensive DNA breakdown. These findings suggest that curtailment of IncIalpha plasmid transfer by the drug results from a general disruption of DNA metabolism rather than from inhibition of a species of RNA essential for transfer.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulnois G. J., Wilkins B. M. A colI-specified product, synthesized in newly infected recipients, limits the amount of DNA transferred during conjugation of Escherichia coli K-12. J Bacteriol. 1978 Jan;133(1):1–9. doi: 10.1128/jb.133.1.1-9.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer H., Churchward G. Deoxyribonucleic acid synthesis after inhibition of initiation of rounds of replication in Escherichia coli B/r. J Bacteriol. 1977 May;130(2):692–697. doi: 10.1128/jb.130.2.692-697.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg S., Griffith J., Kornberg A. phiX174 cistron A protein is a multifunctional enzyme in DNA replication. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3198–3202. doi: 10.1073/pnas.74.8.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkow S., Guerry P., Hedges R. W., Datta N. Polynucleotide sequence relationships among plasmids of the I compatibility complex. J Gen Microbiol. 1974 Nov;85(1):65–76. doi: 10.1099/00221287-85-1-65. [DOI] [PubMed] [Google Scholar]
- Fenwick R. G., Jr, Curtiss R., 3rd Conjugal deoxyribonucleic acid replication by Excherichia coli K-12: effect of chloramphenicol and rifampin. J Bacteriol. 1973 Dec;116(3):1224–1235. doi: 10.1128/jb.116.3.1224-1235.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Datta N. Plasmids determining I pili constitute a compatibility complex. J Gen Microbiol. 1973 Jul;77(1):19–25. doi: 10.1099/00221287-77-1-19. [DOI] [PubMed] [Google Scholar]
- Helmstetter C. E. Initiation of chromosome replication in Escherichia coli. I. Requirements for RNA and protein synthesis at different growth rates. J Mol Biol. 1974 Mar 25;84(1):1–19. doi: 10.1016/0022-2836(74)90209-5. [DOI] [PubMed] [Google Scholar]
- Kingsman A., Willetts N. The requirements for conjugal DNA synthesis in the donor strain during flac transfer. J Mol Biol. 1978 Jul 5;122(3):287–300. doi: 10.1016/0022-2836(78)90191-2. [DOI] [PubMed] [Google Scholar]
- Lark K. G. Evidence for the direct involvement of RNA in the initiation of DNA replication in Escherichia coli 15T. J Mol Biol. 1972 Feb 28;64(1):47–60. doi: 10.1016/0022-2836(72)90320-8. [DOI] [PubMed] [Google Scholar]
- Lawn A. M., Meynell G. G., Meynell E., Datta N. Sex pili and the classification of sex factors in the enterobacteriaceae. Nature. 1967 Oct 28;216(5113):343–346. doi: 10.1038/216343a0. [DOI] [PubMed] [Google Scholar]
- Messer W. Initiation of deoxyribonucleic acid replication in Escherichia coli B-r: chronology of events and transcriptional control of initiation. J Bacteriol. 1972 Oct;112(1):7–12. doi: 10.1128/jb.112.1.7-12.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pato M. L. Alterations of the rate of movement of deoxyribonucleic acid replication forks. J Bacteriol. 1975 Jul;123(1):272–277. doi: 10.1128/jb.123.1.272-277.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabussay D., Zillig W. A rifampicin resistent rna-polymerase from E. coli altered in the beta-subunit. FEBS Lett. 1969 Oct 21;5(2):104–106. doi: 10.1016/0014-5793(69)80305-4. [DOI] [PubMed] [Google Scholar]
- Rowen L., Kornberg A. Primase, the dnaG protein of Escherichia coli. An enzyme which starts DNA chains. J Biol Chem. 1978 Feb 10;253(3):758–764. [PubMed] [Google Scholar]
- Wehrli W., Knüsel F., Schmid K., Staehelin M. Interaction of rifamycin with bacterial RNA polymerase. Proc Natl Acad Sci U S A. 1968 Oct;61(2):667–673. doi: 10.1073/pnas.61.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins B. M., Hollom S. E. Conjugational synthesis of F lac+ and Col I DNA in the presence of rifampicin and in Escherichia coli K12 mutants defective in DNA synthesis. Mol Gen Genet. 1974;134(2):143–156. doi: 10.1007/BF00268416. [DOI] [PubMed] [Google Scholar]
- Wilkins B. M. Partial suppression of the phenotype of Escherichia coli K-12 dnaG mutants by some I-like conjugative plasmids. J Bacteriol. 1975 Jun;122(3):899–904. doi: 10.1128/jb.122.3.899-904.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaritsky A., Pritchard R. H. Replication time of the chromosome in thymineless mutants of Escherichia coli. J Mol Biol. 1971 Aug 28;60(1):65–74. doi: 10.1016/0022-2836(71)90447-5. [DOI] [PubMed] [Google Scholar]



