Abstract
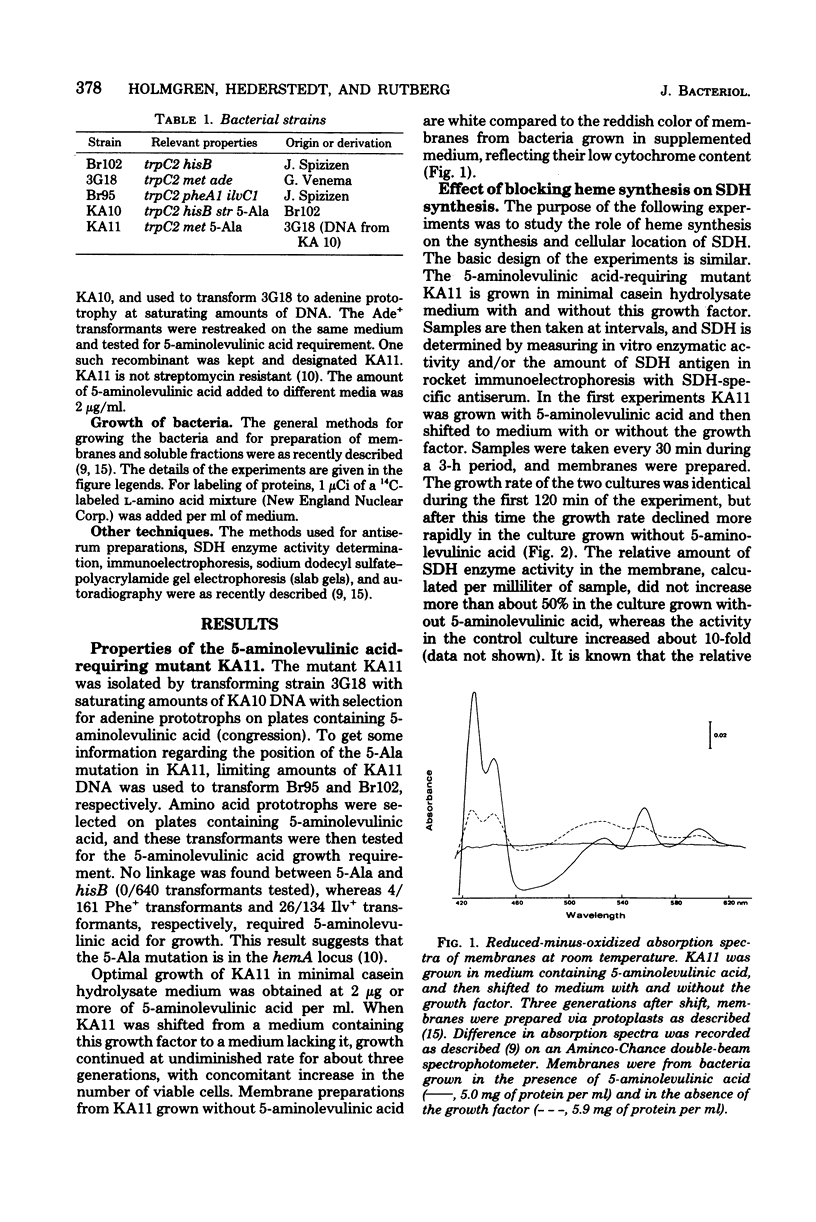
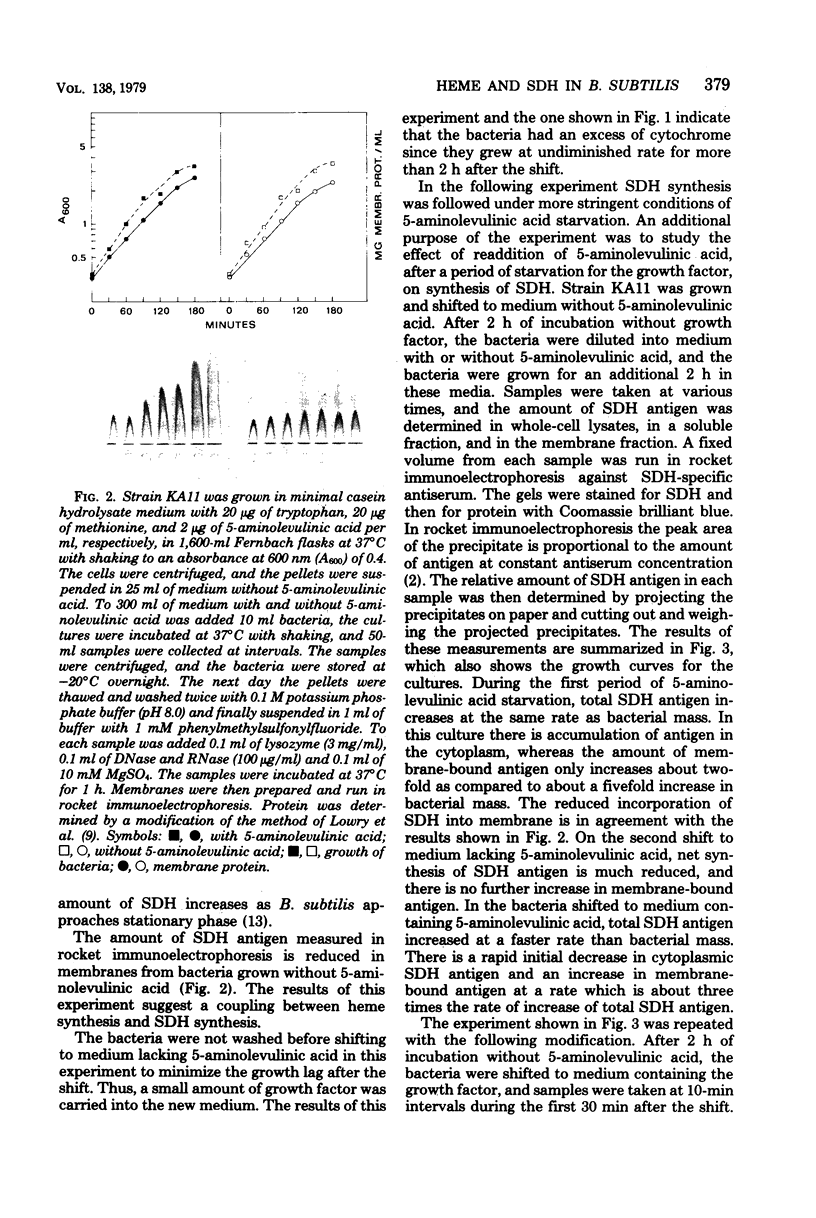
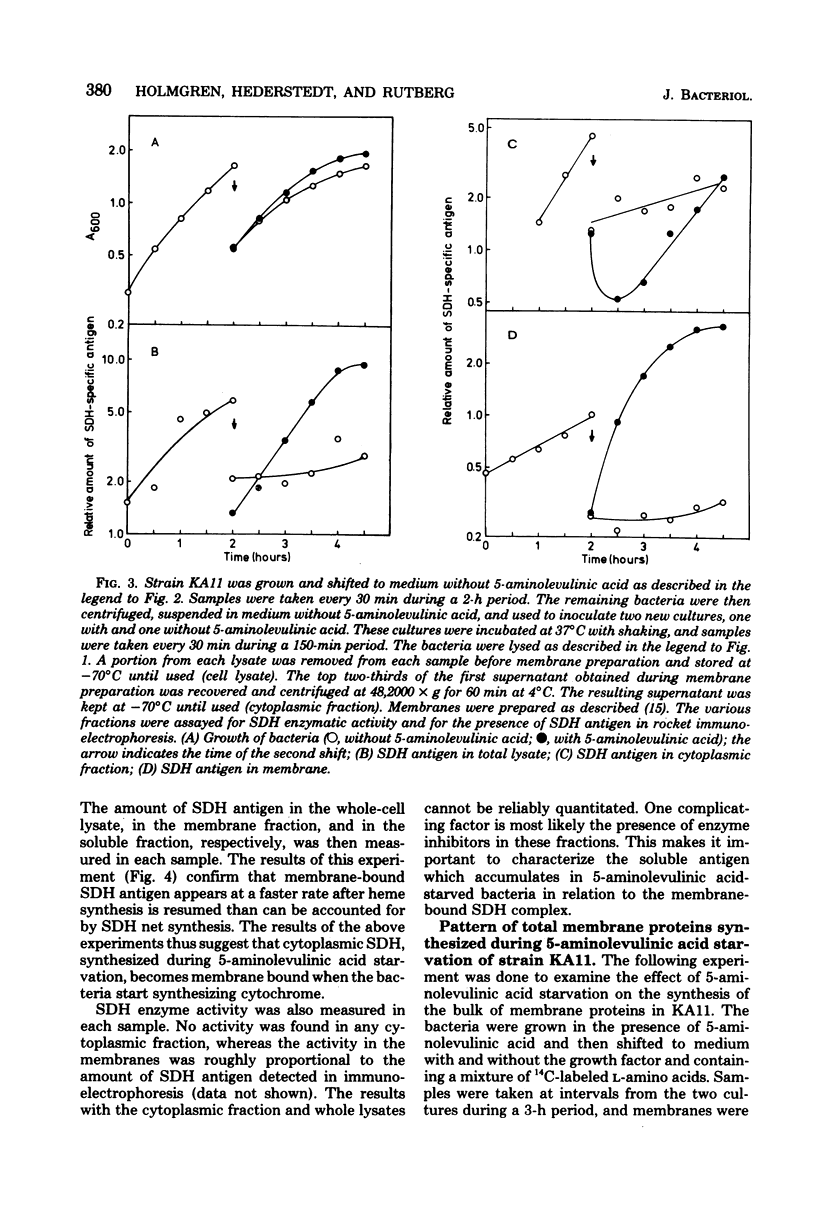
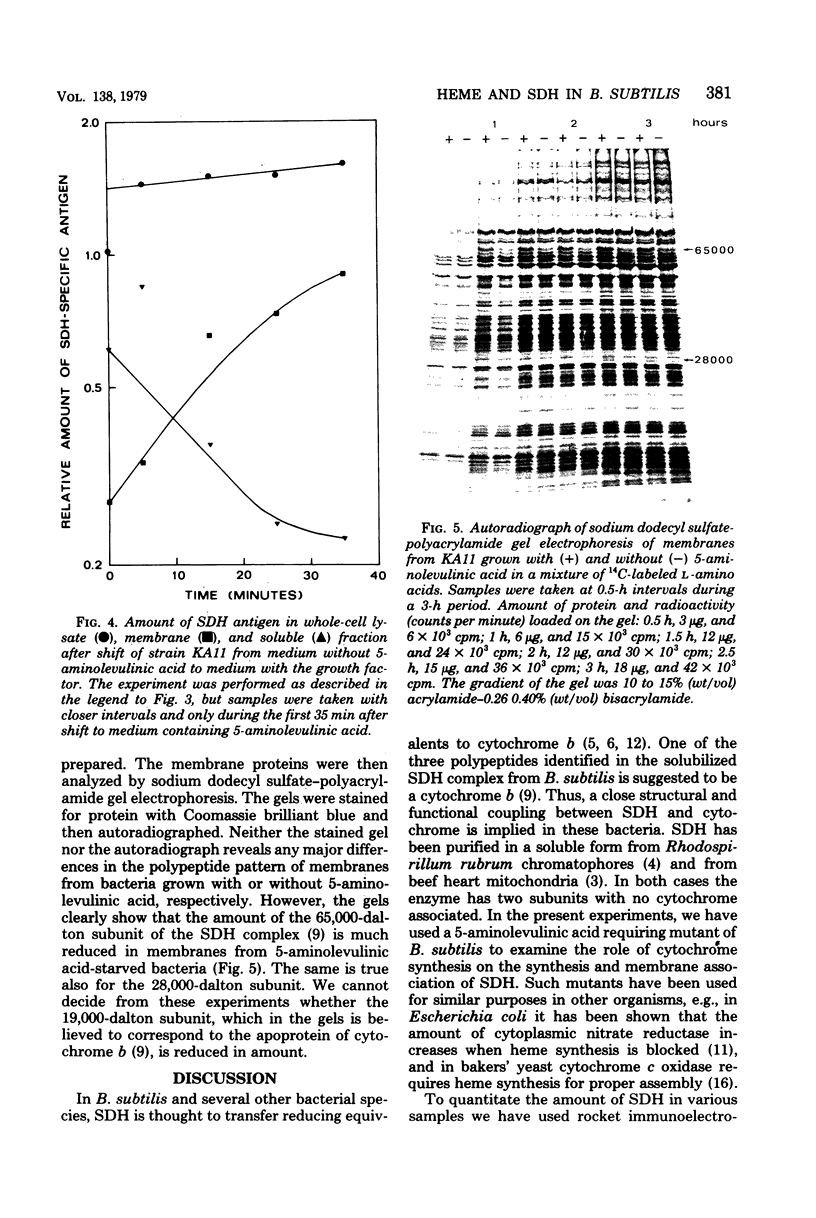
A 5-aminolevulinic acid-requiring mutant of Bacillus subtilis was isolated. When the mutant is shifted from medium containing 5-aminolevulinic acid to medium lacking this growth factor, the bacteria continued to grow at undiminished rate for about three generations. The membranes from these bacteria contained severely reduced amounts of cytochrome. The mutant was used to study the role of heme synthesis on synthesis and membrane binding of succinic dehydrogenase (SDH). The amount of SDH in whole-cell lysates in the soluble cytoplasmic fraction and in membranes was determined by one-dimensional (rocket) immunoelectrophoresis with an SDH-specific antiserum. After heme synthesis was blocked, the relative amount of SDH in the membrane decreased, whereas increasing amounts of SDH antigen were found in the cytoplasm. When heme synthesis was resumed on readdition of 5-aminolevulinic acid, the amount of membrane-bound SDH antigen increased at a much faster rate than net synthesis. During a 3-h growth period without 5-aminolevulinic acid, there was little change in the pattern of membrane proteins as determined by sodium dodecyl sulfate–polyacrylamide gel electrophoresis of radioactively labeled membranes, as compared to membranes from control cultures. However, both the 65,000-dalton and the 28,000-dalton polypeptides of the SDH complex (L. Hederstedt, E. Holmgren, and L. Rutberg, J. Bacteriol. 138:370–376, 1979) were present in decreasing amounts in membranes from 5-aminolevulinic acid-starved bacteria. From these results we suggest that SDH in B. subtilis is synthesized as a soluble protein and becomes membrane bound only when it attaches to a site in the membrane, (part of) which is a cytochrome of b type.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson T. J., Ivánovics G. Isolation and some characteristics of haemin dependent mutants of Bacillus subtilis. J Gen Microbiol. 1967 Oct;49(1):31–40. doi: 10.1099/00221287-49-1-31. [DOI] [PubMed] [Google Scholar]
- Bjerrum O. J. Immunochemical investigation of membrane proteins. A methodological survey with emphasis placed on immunoprecipitation in gels. Biochim Biophys Acta. 1977 Aug 9;472(2):135–195. doi: 10.1016/0304-4157(77)90016-8. [DOI] [PubMed] [Google Scholar]
- Davis K. A., Hatefi Y., Crawford I. P., Baltscheffsky H. Purification, molecular properties, and amino acid composition of the subunits of Rhodospirillum rubrum succinate dehydrogenase. Arch Biochem Biophys. 1977 Apr 30;180(2):459–464. doi: 10.1016/0003-9861(77)90060-1. [DOI] [PubMed] [Google Scholar]
- Davis K. A., Hatefi Y. Succinate dehydrogenase. I. Purification, molecular properties, and substructure. Biochemistry. 1971 Jun 22;10(13):2509–2516. doi: 10.1021/bi00789a014. [DOI] [PubMed] [Google Scholar]
- Farrand S. K., Taber H. W. Physiological effects of menaquinone deficiency in Bacillus subtilis. J Bacteriol. 1973 Sep;115(3):1035–1044. doi: 10.1128/jb.115.3.1035-1044.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanstein W. G., Davis K. A., Ghalambor M. A., Hatefi Y. Succinate dehydrogenase. II. Enzymatic properties. Biochemistry. 1971 Jun 22;10(13):2517–2524. doi: 10.1021/bi00789a015. [DOI] [PubMed] [Google Scholar]
- Hederstedt L., Holmgren E., Rutberg L. Characterization of a succinate dehydrogenase complex solubilized from the cytoplasmic membrane of Bacillus subtilis with the nonionic detergent Triton X-100. J Bacteriol. 1979 May;138(2):370–376. doi: 10.1128/jb.138.2.370-376.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss I., Berek I., Ivánovics G. Mapping the -aminolaevulinic acid synthetase locus in Bacillus subtilis. J Gen Microbiol. 1971 May;66(2):153–159. doi: 10.1099/00221287-66-2-153. [DOI] [PubMed] [Google Scholar]
- MacGregor C. H. Anaerobic cytochrome b1 in Escherichia coli: association with and regulation of nitrate reductase. J Bacteriol. 1975 Mar;121(3):1111–1116. doi: 10.1128/jb.121.3.1111-1116.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohné M. Regulation of the dicarboxylic acid part of the citric acid cycle in Bacillus subtilis. J Bacteriol. 1975 Apr;122(1):224–234. doi: 10.1128/jb.122.1.224-234.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross E., Schatz G. Cytochrome c1 of bakers' yeast. II. Synthesis on cytoplasmic robosomes and influence of oxygen and heme on accumulation of the apoprotein. J Biol Chem. 1976 Apr 10;251(7):1997–2004. [PubMed] [Google Scholar]
- Rutberg B., Hederstedt L., Holmgren E., Rutberg L. Characterization of succinic dehydrogenase mutants of Bacillus subtilis by crossed immunoelectrophoresis. J Bacteriol. 1978 Oct;136(1):304–311. doi: 10.1128/jb.136.1.304-311.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzgaber-Müller J., Schatz G. Heme is necessary for the accumulation and assembly of cytochrome c oxidase subunits in Saccharomyces cerevisiae. J Biol Chem. 1978 Jan 10;253(1):305–310. [PubMed] [Google Scholar]