Abstract
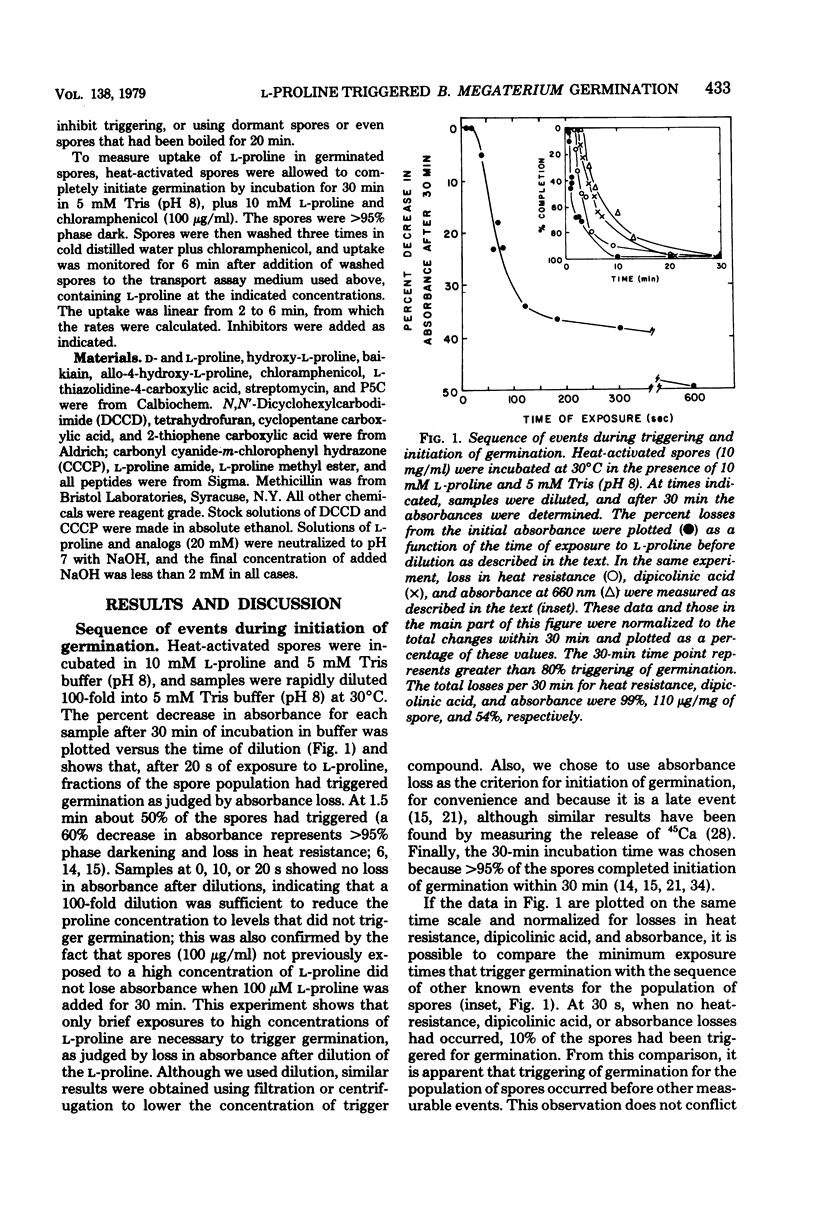
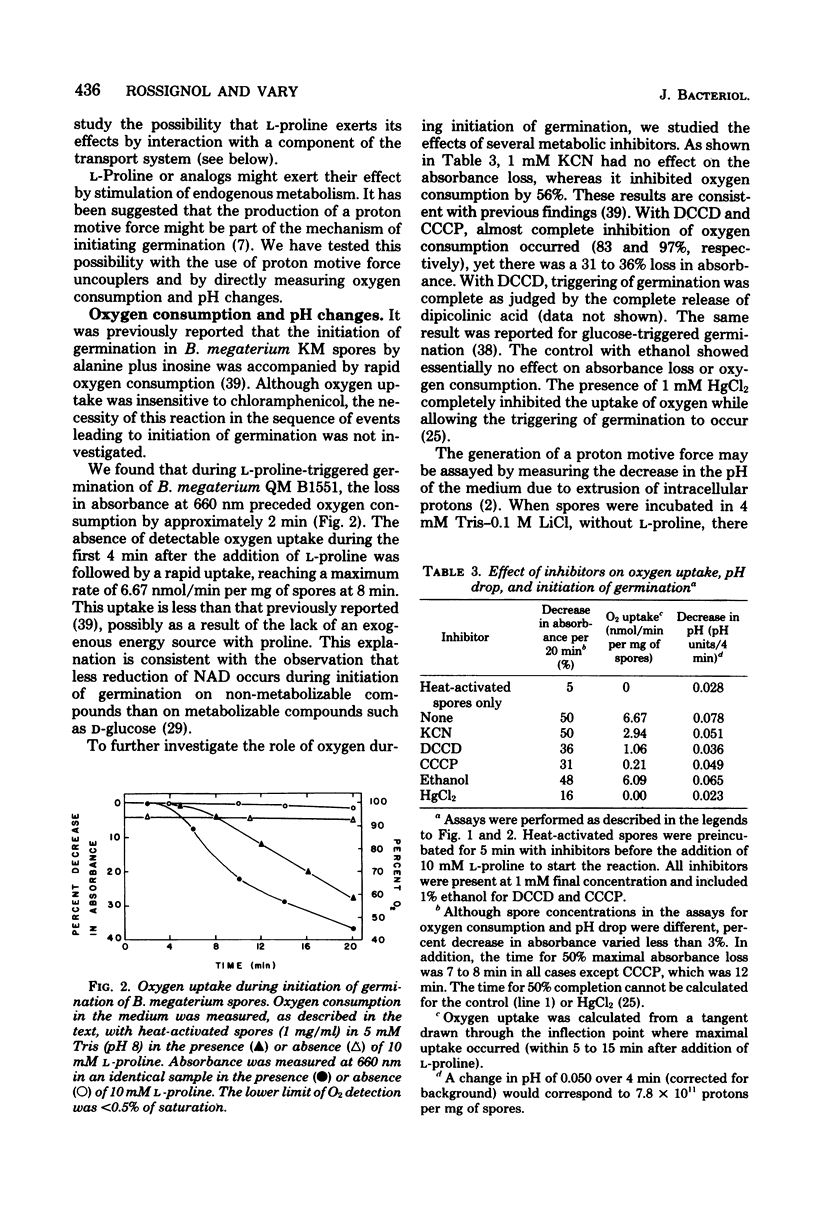
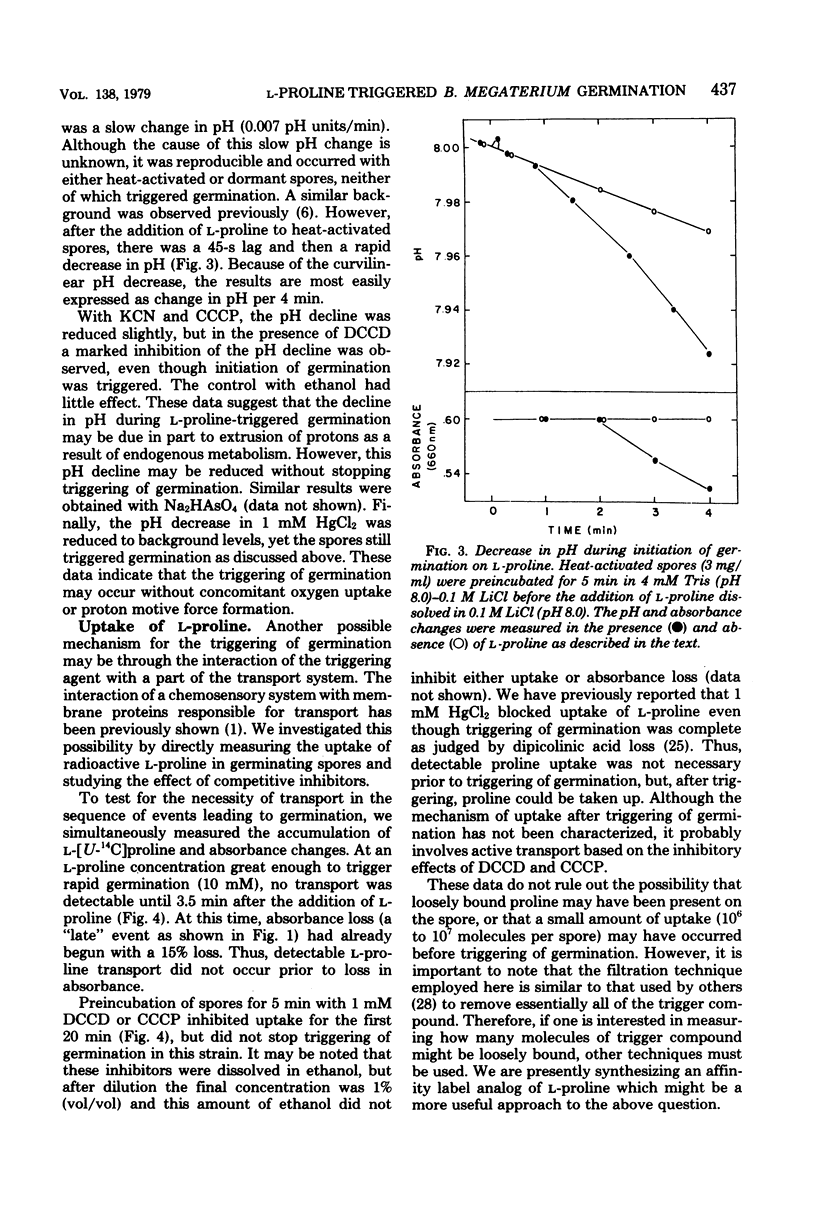
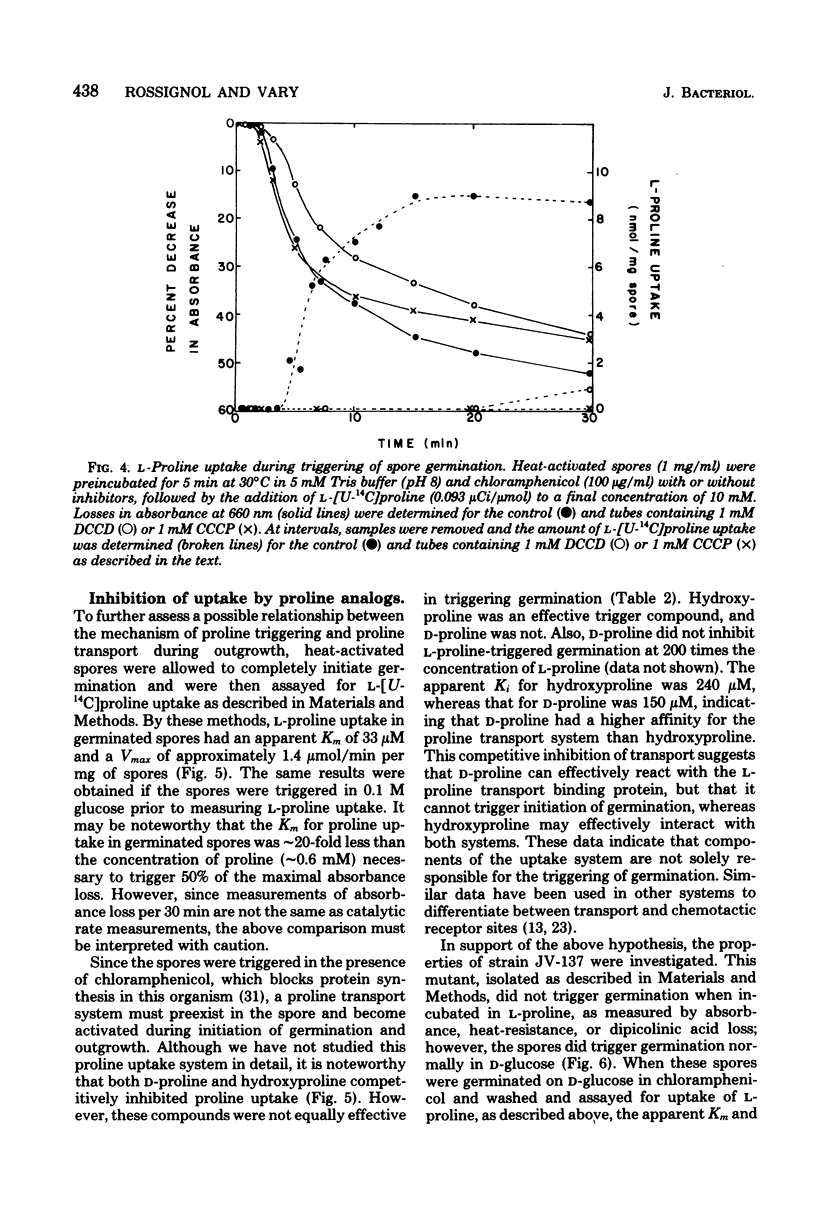
The mechanism by which L-proline triggers germination in Bacillus megaterium QM B1551 spores was investigated. First, brief exposure of spores to L-proline, followed by dilution, was sufficient to trigger germination. Once germination was triggered, the spores continued initiation of germination and did not require high concentrations of L-proline. Triggering of germination was pH and temperature dependent. Second, enzymes for L-proline catabolism were absent in spores, and several non-metabolizable analogs of L-proline were effective trigger compounds. Third, triggering of germination occurred in the presence of inhibitors of proton motive force production, oxygen uptake, and metabolism. Fourth, uptake of L-proline occurred after the triggering of germination. These results argue that neither uptake nor metabolism of L-proline was necessary to trigger germination. Instead, L-proline probably causes a biophysical alteration in the spores that triggers the biochemical changes in germination.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. Chemotaxis in bacteria. Annu Rev Biochem. 1975;44:341–356. doi: 10.1146/annurev.bi.44.070175.002013. [DOI] [PubMed] [Google Scholar]
- Costilow R. N., Cooper D. Identity of proline dehydrogenase and delta1-pyrroline-5-carboxylic acid reductase in Clostridium sporogenes. J Bacteriol. 1978 Apr;134(1):139–146. doi: 10.1128/jb.134.1.139-146.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dendinger S., Brill W. J. Effect of the proline analogue baikiain on proline metabolism in Salmonella typhimurium. J Bacteriol. 1972 Dec;112(3):1134–1141. doi: 10.1128/jb.112.3.1134-1141.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dendinger S., Brill W. J. Regulation of proline degradation in Salmonella typhimurium. J Bacteriol. 1970 Jul;103(1):144–152. doi: 10.1128/jb.103.1.144-152.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dills S. S., Vary J. C. An evaluation of respiration chain-associated functions during initiation of germination of Bacillus megaterium spores. Biochim Biophys Acta. 1978 Jul 3;541(3):301–311. doi: 10.1016/0304-4165(78)90190-3. [DOI] [PubMed] [Google Scholar]
- FRANK L., RANHAND B. PROLINE METABOLISM IN ESCHERICHIA COLI. 3. THE PROLINE CATABOLIC PATHWAY. Arch Biochem Biophys. 1964 Aug;107:325–331. doi: 10.1016/0003-9861(64)90338-8. [DOI] [PubMed] [Google Scholar]
- HALMANN M., KEYNAN A. Stages in germination of spores of Bacillus licheniformis. J Bacteriol. 1962 Dec;84:1187–1193. doi: 10.1128/jb.84.6.1187-1193.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRELL W. K., HALVORSON H. Studies on the role of L-alanine in the germination of spores of Bacillus terminalis. J Bacteriol. 1955 Mar;69(3):275–279. doi: 10.1128/jb.69.3.275-279.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HYATT M. T., LEVINSON H. S. EFFECT OF SUGARS AND OTHER CARBON COMPOUNDS ON GERMINATION AND POSTGERMINATIVE DEVELOPMENT OF BACILLUS MEGATERIUM SPORES. J Bacteriol. 1964 Nov;88:1403–1415. doi: 10.1128/jb.88.5.1403-1415.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansford R. G., Sacktor B. The control of the oxidation of proline by isolated flight muscle mitochondria. J Biol Chem. 1970 Mar 10;245(5):991–994. [PubMed] [Google Scholar]
- Hazelbauer G. L., Adler J. Role of the galactose binding protein in chemotaxis of Escherichia coli toward galactose. Nat New Biol. 1971 Mar 24;230(12):101–104. doi: 10.1038/newbio230101a0. [DOI] [PubMed] [Google Scholar]
- Hsieh L. K., Vary J. C. Germination and peptidoglycan solubilization in Bacillus megaterium spores. J Bacteriol. 1975 Aug;123(2):463–470. doi: 10.1128/jb.123.2.463-470.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINSON H. S., HYATT M. T. Nitrogenous compounds in germination and postgerminative development of Bacillus megaterium spores. J Bacteriol. 1962 Jun;83:1224–1230. doi: 10.1128/jb.83.6.1224-1230.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laishley E. J., Bernlohr R. W. Regulation of arginine and proline catabolism in Bacillus licheniformis. J Bacteriol. 1968 Aug;96(2):322–329. doi: 10.1128/jb.96.2.322-329.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson H. S., Hyatt M. T. Sequence of events during Bacillus megaterim spore germination. J Bacteriol. 1966 May;91(5):1811–1818. doi: 10.1128/jb.91.5.1811-1818.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordal G. W., Villani D. P., Nicholas R. A., Hamel F. G. Independence of proline chemotaxis and transport in Bacillus subtilis. J Biol Chem. 1978 Jul 25;253(14):4916–4919. [PubMed] [Google Scholar]
- Prasad C., Diesterhaft M., Freese E. Initiation of spore germination in glycolytic mutants of Bacillus subtilis. J Bacteriol. 1972 Apr;110(1):321–328. doi: 10.1128/jb.110.1.321-328.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol D. P., Vary J. C. A unique method for studying the initiation of Bacillus megaterium spore germination. Biochem Biophys Res Commun. 1977 Dec 21;79(4):1098–1103. doi: 10.1016/0006-291x(77)91118-4. [DOI] [PubMed] [Google Scholar]
- Scott I. R., Ellar D. J. Study of calcium dipicolinate release during bacterial spore germination by using a new, sensitive assay for dipicolinate. J Bacteriol. 1978 Jul;135(1):133–137. doi: 10.1128/jb.135.1.133-137.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B., Setlow P. Levels of oxidized and reduced pyridine nucleotides in dormant spores and during growth, sporulation, and spore germination of Bacillus megaterium. J Bacteriol. 1977 Feb;129(2):857–865. doi: 10.1128/jb.129.2.857-865.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Biochemical studies of bacterial sporulation and germination. 23. Nucleotide metabolism during spore germination. J Biol Chem. 1970 Jul 25;245(14):3645–3652. [PubMed] [Google Scholar]
- Setlow P., Primus G. Protein metabolism during germination of Bacillus megaterium spores. I. Protein synthesis and amino acid metabolism. J Biol Chem. 1975 Jan 25;250(2):623–630. [PubMed] [Google Scholar]
- Setlow P. Protein metabolism during germination of Bacillus megaterium spores. II. Degradation of pre-existing and newly synthesized protein. J Biol Chem. 1975 Jan 25;250(2):631–637. [PubMed] [Google Scholar]
- Seto B., Stadtman T. C. Purification and properties of proline reductase from Clostridium sticklandii. J Biol Chem. 1976 Apr 25;251(8):2435–2439. [PubMed] [Google Scholar]
- Shay L. K., Vary J. C. Biochemical studies on glucose initiated germination in Bacillus megaterium. Biochim Biophys Acta. 1978 Jan 18;538(2):284–292. doi: 10.1016/0304-4165(78)90356-2. [DOI] [PubMed] [Google Scholar]
- Van der Werf P., Orlowski M., Meister A. Enzymatic conversion of 5-oxo-L-proline (L-pyrrolidone carboxylate) to L-glutamate coupled with cleavage of adenosine triphosphate to adenosine diphosphate, a reaction in the -glutamyl cycle. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2982–2985. doi: 10.1073/pnas.68.12.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vary J. C. Spore germination of Bacillus megaterium QM B1551 mutants. J Bacteriol. 1972 Oct;112(1):640–642. doi: 10.1128/jb.112.1.640-642.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B. J., Ellar D. J., Scott I. R., Koncewicz M. A. Rapid, chloramphenicol-resistant, activation of membrane electron transport on germination of Bacillus spores. Nature. 1977 Mar 10;266(5598):174–176. doi: 10.1038/266174a0. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Vary J. C., Halvorson H. O. A kinetic model for bacterial spore germination. Proc Natl Acad Sci U S A. 1968 Mar;59(3):869–875. doi: 10.1073/pnas.59.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]


