Abstract
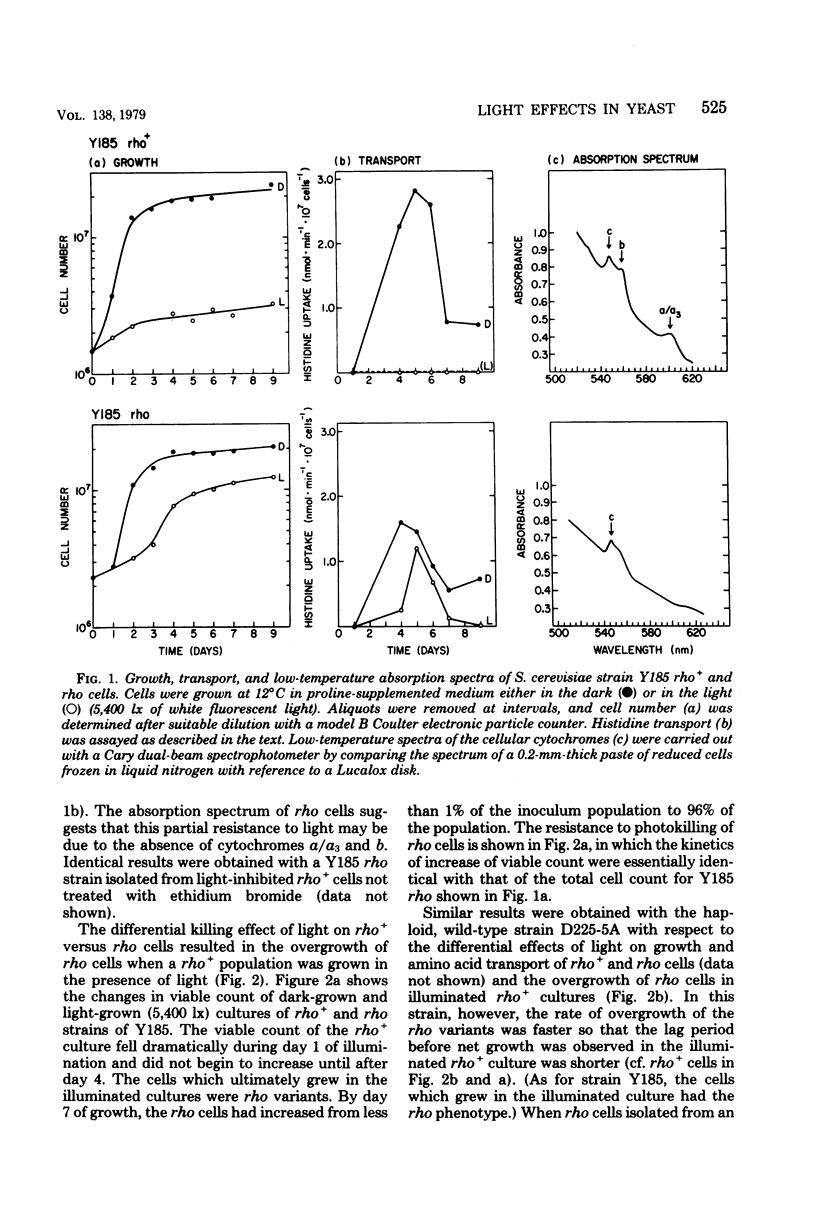
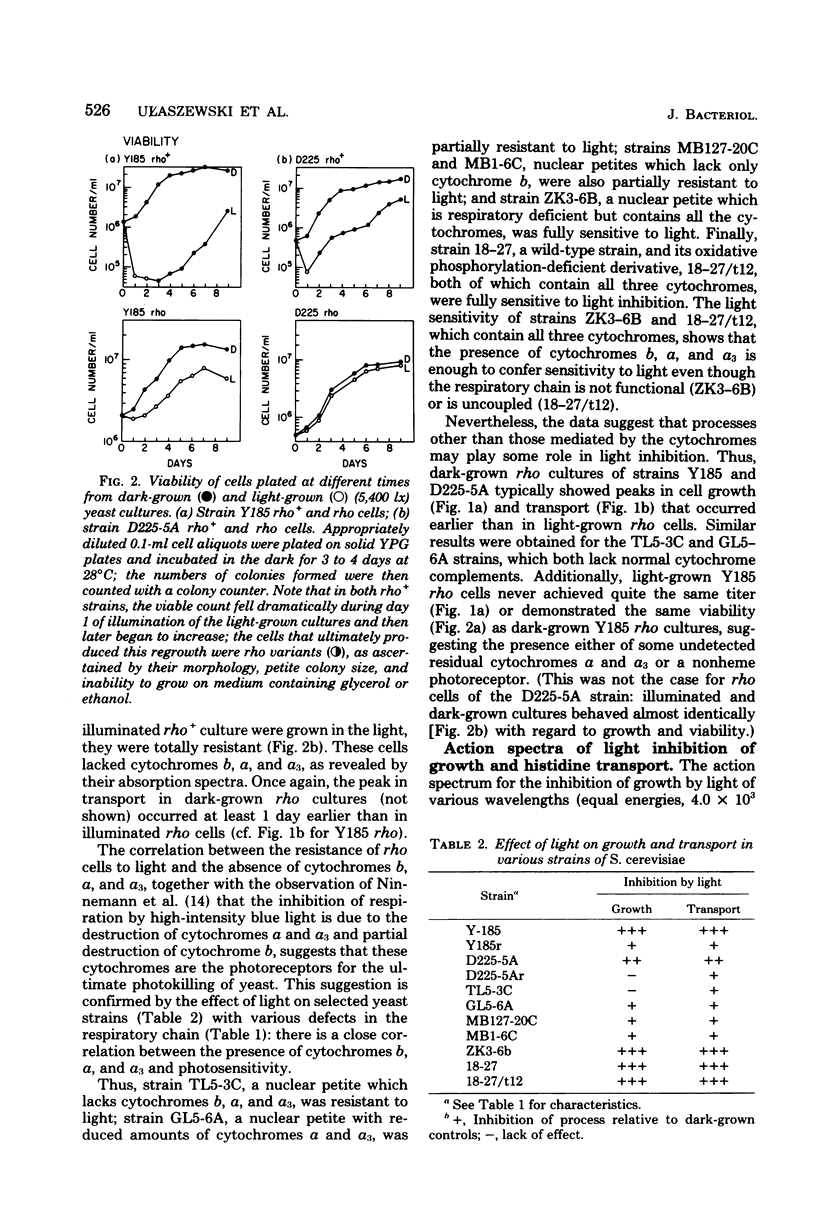
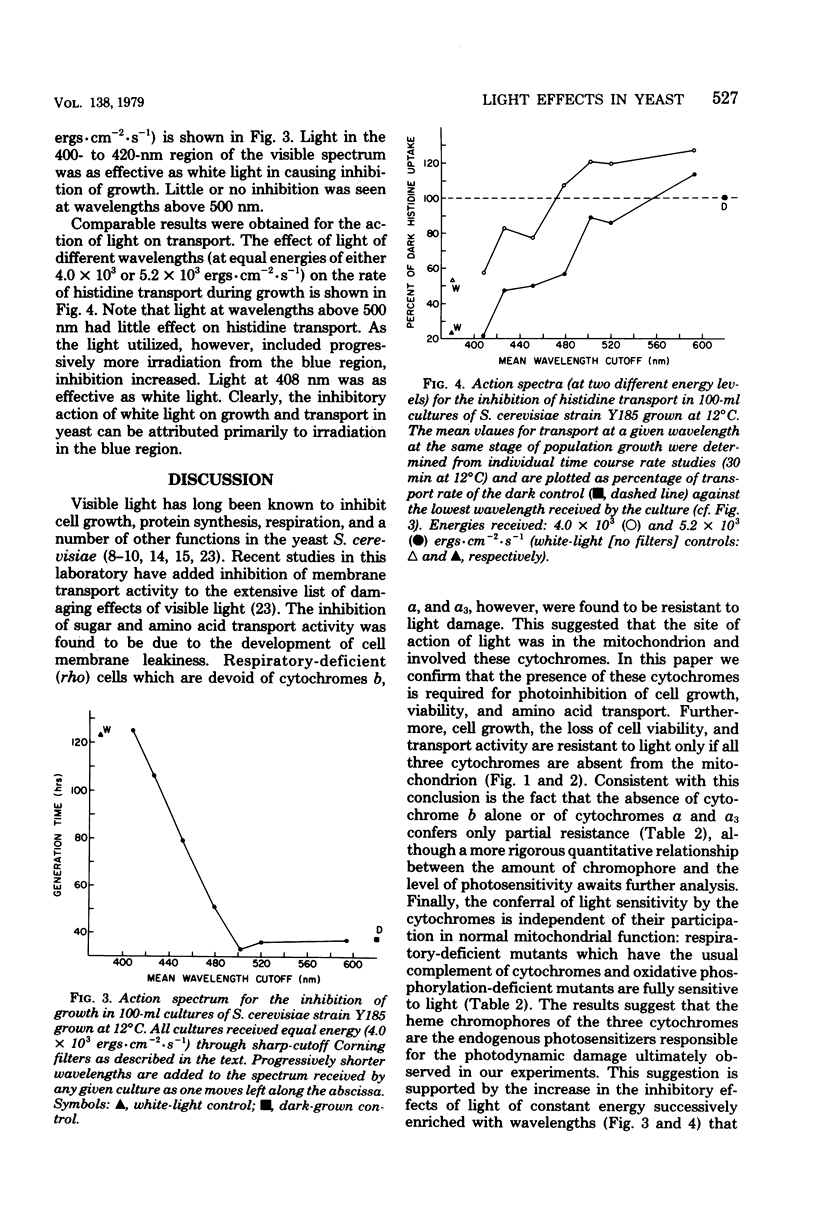
Visible light of moderate intensity inhibits growth, respiration, protein synthesis, and membrane transport in bakers' yeast and has a deleterious effect on membrane integrity. The results of this study indicate that these effects require the presence of cytochromes b and a/a3. The light sensitivities of growth rate and [14C]histidine uptake in wild-type rho+ Y185 and D225-5A strains of Saccharomyces cerevisiae were compared with those in a variety of mutants lacking cytochrome b or a/a3 or both; a close correlation was found between the presence of these respiratory pigments and photosensitivity. Thus, strain TL5-3C, a nuclear petite lacking cytochromes b, a, and a3, was resistant to light; strain GL5-6A, another nuclear petite having reduced amounts of cytochromes a and a3, was partially resistant; strains MB127-20C and MB1-6C, nuclear petites lacking only cytochrome b, were also only partially resistant to light; whereas mutants containing all three cytochromes but having their respiratory chain either nonfunctional (strain ZK3-6B) or uncoupled (strain 18-27/t12) were fully sensitive to light. Finally, an equal-energy, broad-band action spectrum for the light inhibition of growth and transport indicated that blue light (408 nm) was most effective; these wavelengths correspond to the Soret region of the cytochrome absorption spectrum. The results suggest, therefore, that the yeast cytochromes b, a, and a3 are the primary photoreceptors for the inhibitory effects of light and, perhaps, for other processes, such as the entrainment of biological rhythms in this species.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal B. B., Avi-Dor Y., Tinberg H. M., Packer L. Effect of visible light on the mitochondrial inner membrane. Biochem Biophys Res Commun. 1976 Mar 22;69(2):362–368. doi: 10.1016/0006-291x(76)90530-1. [DOI] [PubMed] [Google Scholar]
- Doyle R. J., Kubitschek H. E. Near ultraviolet light inactivation of an energy-independent membrane transport system in Saccharomyces cerevisiae. Photochem Photobiol. 1976 Sep;24(3):291–293. doi: 10.1111/j.1751-1097.1976.tb06825.x. [DOI] [PubMed] [Google Scholar]
- Edmunds L. N., Jr, Cirillo V. P. On the interplay among cell cycle, biological clock and membrane transport control systems. Int J Chronobiol. 1974;2(3):233–246. [PubMed] [Google Scholar]
- Ehrenberg M. Wirjungen sichtbaren Lichtes auf Saccharomyces cerevisiae. II. Die Gärung unter den Einfluss von Pasteur-Effekt und Licht-Effekt. Arch Mikrobiol. 1966 Oct 19;55(1):26–30. [PubMed] [Google Scholar]
- Epel B. L. Inhibition of growth and respiration by visible and near-visible light. Photophysiology. 1973;0(0):209–229. doi: 10.1016/b978-0-12-282608-5.50013-8. [DOI] [PubMed] [Google Scholar]
- Guerin B., Jacques R. Photoinhibition de l'adaptation respiratoire chez Saccharomyces cerevisiae. II. Le spectre d'action. Biochim Biophys Acta. 1968 Jan 15;153(1):138–142. doi: 10.1016/0005-2728(68)90154-0. [DOI] [PubMed] [Google Scholar]
- Guerin B., Sulkowski E. Photoinhibition de l'adaptation respiratoire chez Saccharomyces cerevisiae. I. Variations de la sensibilite à l'inhibition. Biochim Biophys Acta. 1966 Oct 24;129(1):193–200. [PubMed] [Google Scholar]
- Koch A. L., Doyle R. J., Kubitschek H. E. Inactivation of membrane transport in Escherichia coli by near-ultraviolet light. J Bacteriol. 1976 Apr;126(1):140–146. doi: 10.1128/jb.126.1.140-146.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachowicz T. M., Kotylak Z., Kolodyński J., Sniegocka Z. New types of respiratory deficient mutants in Saccharomyces cerevisiae. II. Physiology and genetics of a series of segregational mutants induced by ultraviolet irradiation or nitrous acid treatment. Arch Immunol Ther Exp (Warsz) 1969;17(1):72–85. [PubMed] [Google Scholar]
- Lachowicz T. M. New types of respiratory deficient mutants in Saccharomyces cerevisiae. I. A segregational mutant with ineffective respiration. Arch Immunol Ther Exp (Warsz) 1968;16(5):693–701. [PubMed] [Google Scholar]
- Ninnemann H., Butler W. L., Epel B. L. Inhibition of respiration and destruction of cytochrome A3 by light in mitochondria and cytochrome oxidase from beef heart. Biochim Biophys Acta. 1970 Jun 30;205(3):507–512. doi: 10.1016/0005-2728(70)90116-7. [DOI] [PubMed] [Google Scholar]
- Ninnemann H., Butler W. L., Epel B. L. Inhibition of respiration in yeast by light. Biochim Biophys Acta. 1970 Jun 30;205(3):499–506. doi: 10.1016/0005-2728(70)90115-5. [DOI] [PubMed] [Google Scholar]
- Ninnemann H., Strasser R. J., Butler W. L. The superoxide anion as electron donor to the mitochondrial electron transport chain. Photochem Photobiol. 1977 Jul;26(1):41–47. doi: 10.1111/j.1751-1097.1977.tb07446.x. [DOI] [PubMed] [Google Scholar]
- Subík J., Kuzela S., Kolarov J., Kovác L., Lachowicz T. M. Oxidative phosphorylation in yeast. VI. ATPase activity and protein synthesis in mitochondria isolated from nuclear mutants deficient in cytochromes. Biochim Biophys Acta. 1970 Jun 30;205(3):513–519. doi: 10.1016/0005-2728(70)90117-9. [DOI] [PubMed] [Google Scholar]
- Ulaszewski S., Lachowicz T. M. Genetic modification of cytochrome b deficient mutants in Saccharomyces cerevisiae. Mol Gen Genet. 1974;131(1):69–77. doi: 10.1007/BF00269388. [DOI] [PubMed] [Google Scholar]
- Ulaszewski S., Lachowicz T. M. Specificity and origin of the Mod2- mutation which restores respiratory sufficient phenotype in some pet mutants in Saccharomyces cerevisiae. Acta Microbiol Pol A. 1975;7(2):77–85. [PubMed] [Google Scholar]
- Ulaszewski S., Woodward J. R., Cirillo V. P. Membrane damage associated with inositol-less death in Saccharomyces cerevisiae. J Bacteriol. 1978 Oct;136(1):49–54. doi: 10.1128/jb.136.1.49-54.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickerham L. J. A Critical Evaluation of the Nitrogen Assimilation Tests Commonly Used in the Classification of Yeasts. J Bacteriol. 1946 Sep;52(3):293–301. [PMC free article] [PubMed] [Google Scholar]
- Woodward J. R., Cirillo V. P. Amino acid transport and metabolism in nitrogen-starved cells of Saccharomyces cerevisiae. J Bacteriol. 1977 May;130(2):714–723. doi: 10.1128/jb.130.2.714-723.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward J. R., Cirillo V. P., Edmunds L. N., Jr Light effects in yeast: inhibition by visible light of growth and transport in Saccharomyces cerevisiae grown at low temperatures. J Bacteriol. 1978 Feb;133(2):692–698. doi: 10.1128/jb.133.2.692-698.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



