Abstract
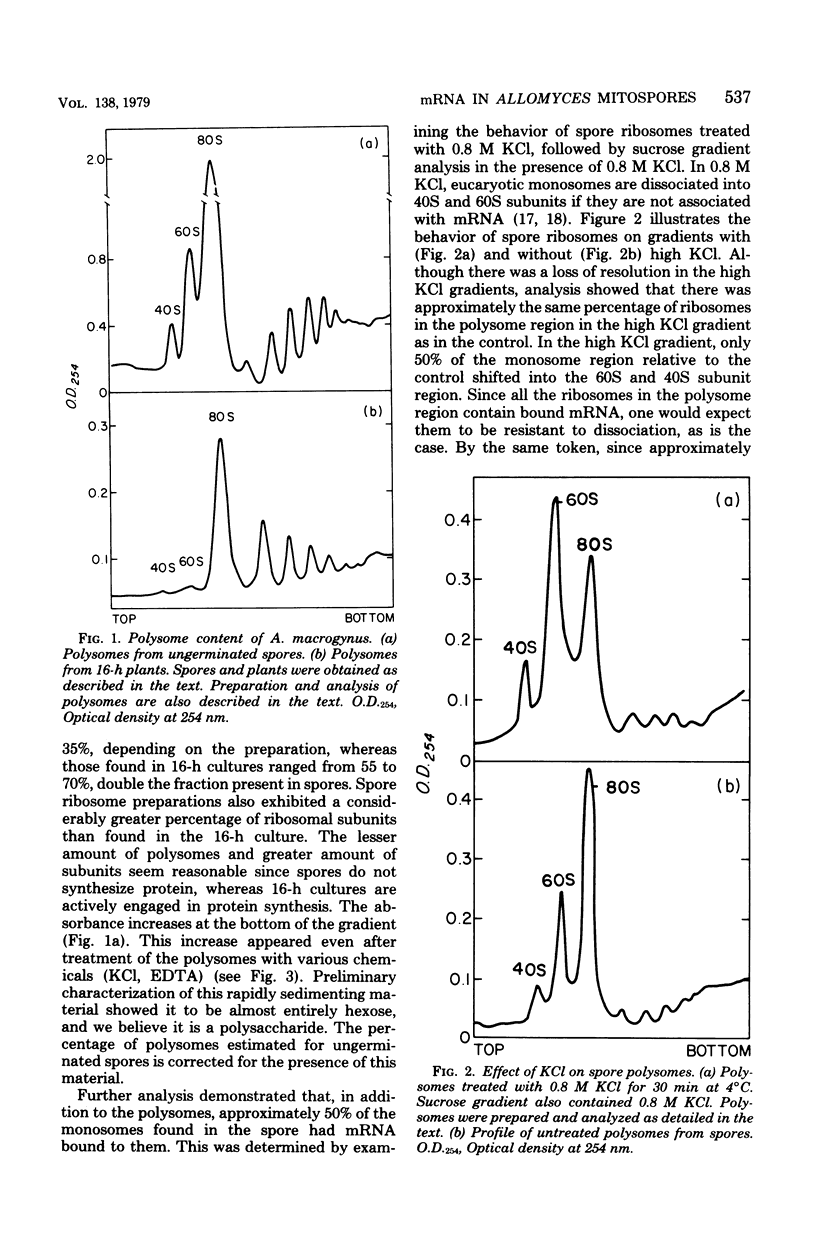
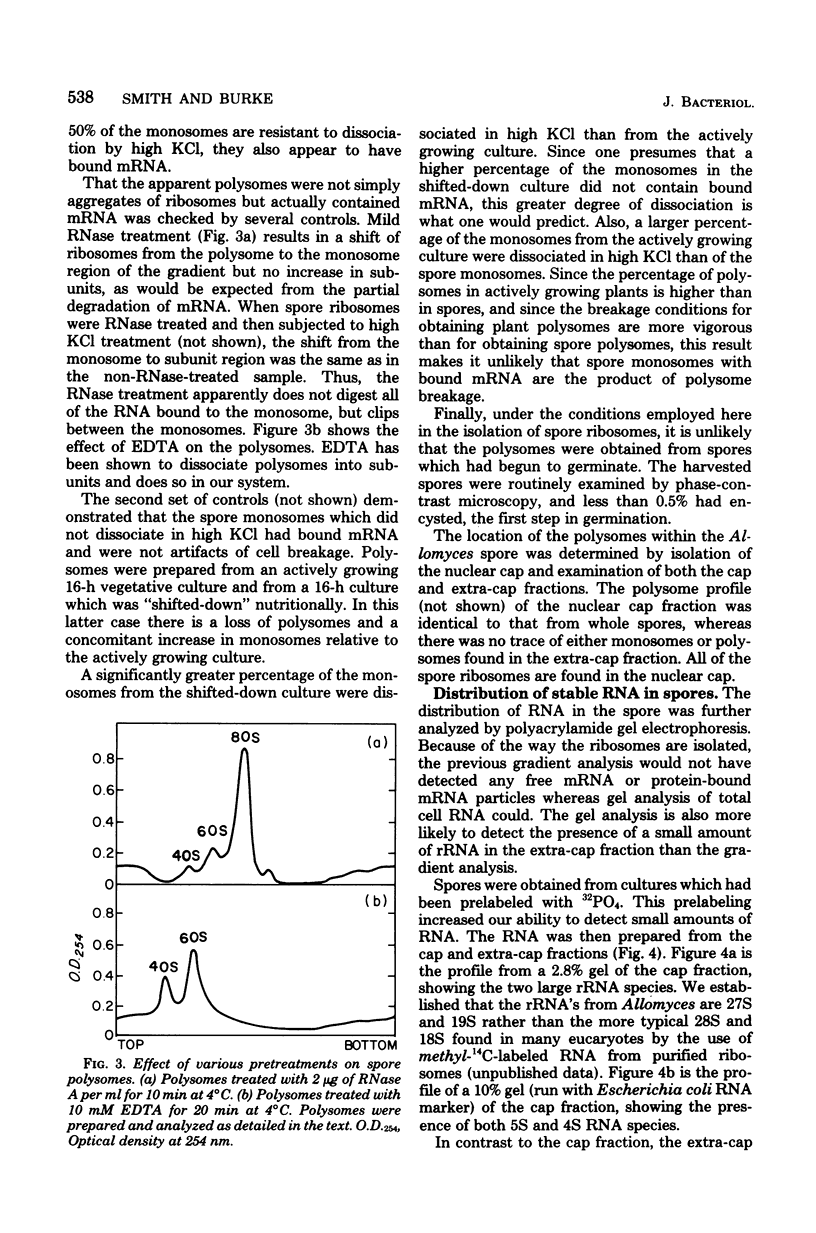
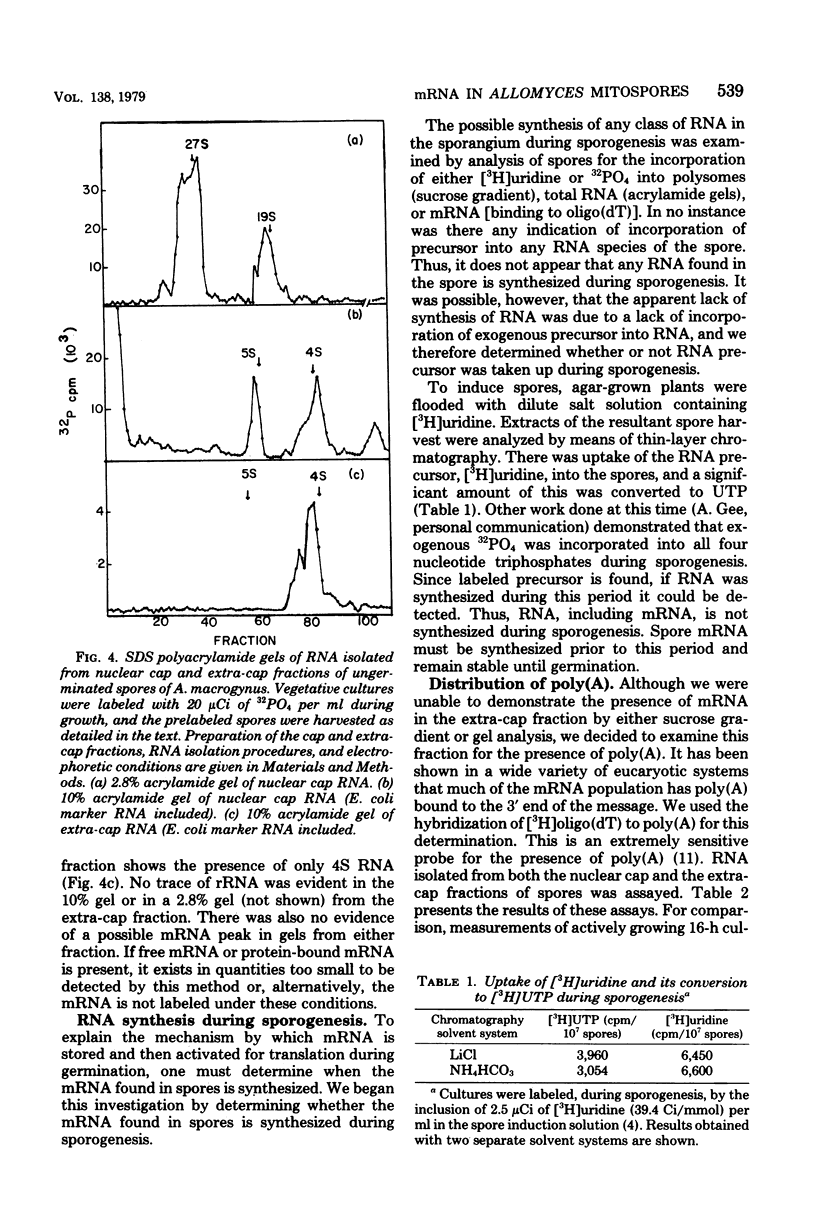
Sucrose density gradient analysis was used to show that polysomes were present in the mitospores of Allomyces macrogynus. Fifty percent of the spore monosomes were shown to be resistant to dissociation by 0.8 M KCl, indicating that messenger ribonucleic acid (mRNA) was bound to them. These polysomes and all the spore ribosomes were contained in the nuclear cap. Only 4S RNA could be demonstrated in the extra-cap fraction. Hybridization studies using 3H-labeled polydeoxythymidylic acid indicated that polyadenylate was present to the extent of 0.08% of the total spore RNA. Sixty-eight percent of the polyadenylic acid is found in the nuclear cap, and 32% is found in the extra-cap fraction. It was demonstrated that [3H]uridine was taken up by the spores and converted to uridine triphosphate. Lack of incorporation of 3H into RNA indicated that the spores do not synthesize RNA. Thus, the mRNA found in spores is synthesized prior to spore formation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avadhani N. G., Buetow D. E. Isolation of active polyribosomes from the cytoplasm, mitochondria and chloroplasts of Euglena gracilis. Biochem J. 1972 Jun;128(2):353–365. doi: 10.1042/bj1280353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambl R. M., Van Etten J. L. Protein synthesis during fungal spore germination. V. Evidence that the ungerminated conidiospores of Botryodiplodia theobromae contain messenger ribonucleic acid. Arch Biochem Biophys. 1970 Apr;137(2):442–452. doi: 10.1016/0003-9861(70)90461-3. [DOI] [PubMed] [Google Scholar]
- Brambl R. Presence of polyribosomes in condiospores of Botryodiplodia theobromae harvested with nonaqueous solvents. J Bacteriol. 1975 Jun;122(3):1394–1395. doi: 10.1128/jb.122.3.1394-1395.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D. J., Seale T. W., McCarthy B. J. Protein and ribonucleic acid synthesis during the diploid life cycle of Allomyces arbuscula. J Bacteriol. 1972 Jun;110(3):1065–1072. doi: 10.1128/jb.110.3.1065-1072.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firtel R. A., Jacobson A., Lodish H. F. Isolation and hybridization kinetics of messenger RNA from Dictyostelium discoideum. Nat New Biol. 1972 Oct 25;239(95):225–228. doi: 10.1038/newbio239225a0. [DOI] [PubMed] [Google Scholar]
- Hollomon D. W. Biochemistry of germination in Peronospora tabacina (Adam) conidia: evidence for the existence of stable messenger RNA. J Gen Microbiol. 1969 Feb;55(2):267–274. doi: 10.1099/00221287-55-2-267. [DOI] [PubMed] [Google Scholar]
- Jaworski A. J. Synthesis of polyadenylic acid RNA during zoospore differentiation and germination in Blastocladiella emersonii. Arch Biochem Biophys. 1976 Mar;173(1):201–209. doi: 10.1016/0003-9861(76)90250-2. [DOI] [PubMed] [Google Scholar]
- Johnson S. A., Lovett J. S., Wilt F. H. The polyadenylated RNA of zoospores and growth phase cells of the aquatic fungus, Blastocladiella. Dev Biol. 1977 Apr;56(2):329–342. doi: 10.1016/0012-1606(77)90274-3. [DOI] [PubMed] [Google Scholar]
- Kaufman S. J., Gross K. W. Quantitation and size determination of poly(A) by hybridization to (3H)poly(dT). Biochim Biophys Acta. 1974 Jun 27;353(2):133–145. doi: 10.1016/0005-2787(74)90180-4. [DOI] [PubMed] [Google Scholar]
- Knight R. H., Van Etten J. L. Characteristics of ribonucleic acids isolated for Botryodiplodia theobromae pycnidiospores. Arch Microbiol. 1976 Aug;109(1-2):45–50. doi: 10.1007/BF00425111. [DOI] [PubMed] [Google Scholar]
- LOVETT J. S. CHEMICAL AND PHYSICAL CHARACTERIZATION OF "NUCLEAR CAPS" ISOLATED FROM BLASTOCLADIELLA ZOOSPORES. J Bacteriol. 1963 Jun;85:1235–1246. doi: 10.1128/jb.85.6.1235-1246.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver C. J., Lovett J. S. An analysis of protein and RNA synthesis during encystment and outgrowth (germination) of Blastocladiella zoospores. Cell Differ. 1974 Sep;3(3):165–192. doi: 10.1016/0045-6039(74)90028-1. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T. E., Hartwell L. H. Resistance of active yeast ribosomes to dissociation by KCl. J Biol Chem. 1970 Mar 25;245(6):1504–1506. [PubMed] [Google Scholar]
- Martin T. E., Wool I. G. Active hybrid 80 s particles formed from subunits of rat, rabbit and protozoan (Tetrahymena pyriformis) ribosomes. J Mol Biol. 1969 Jul 14;43(1):151–161. doi: 10.1016/0022-2836(69)90085-0. [DOI] [PubMed] [Google Scholar]
- Mirkes P. E., McCalley B. Synthesis of polyadenylic acid-containing ribonucleic acid during the germination of Neurospora crassa conidia. J Bacteriol. 1976 Jan;125(1):174–180. doi: 10.1128/jb.125.1.174-180.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirkes P. E. Polysomes, ribonucleic acid, and protein synthesis during germination of Neurospora crassa conidia. J Bacteriol. 1974 Jan;117(1):196–202. doi: 10.1128/jb.117.1.196-202.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roheim J. R., Knight R. H., van Etten J. L. Synthesis of ribonucleic acids during the germination of Rhizopus stolonifer sporangiospores. Dev Biol. 1974 Nov;41(1):137–145. doi: 10.1016/0012-1606(74)90289-9. [DOI] [PubMed] [Google Scholar]
- Sagher D., Edelman M., Jakob K. M. Poly(A)-associated RNA in plants. Biochim Biophys Acta. 1974 Apr 27;349(1):32–38. doi: 10.1016/0005-2787(74)90005-7. [DOI] [PubMed] [Google Scholar]



