Abstract
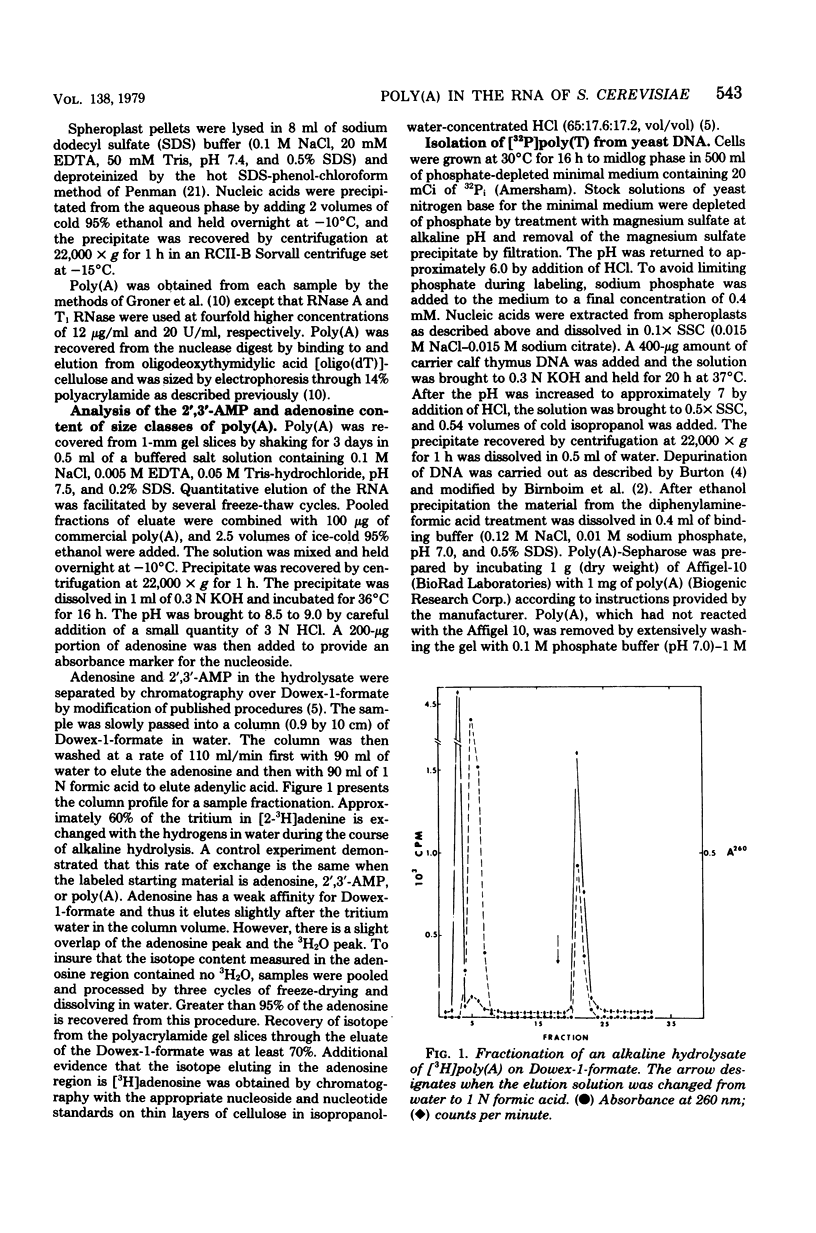
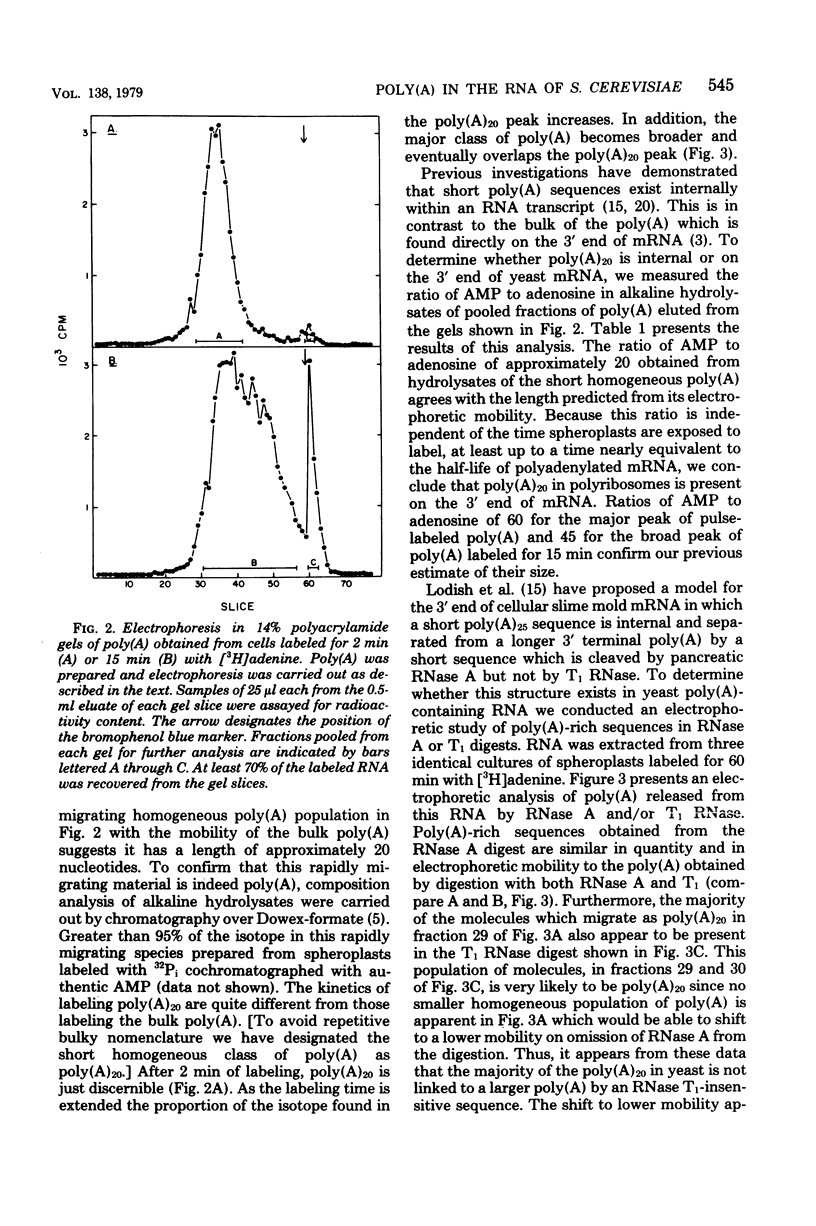
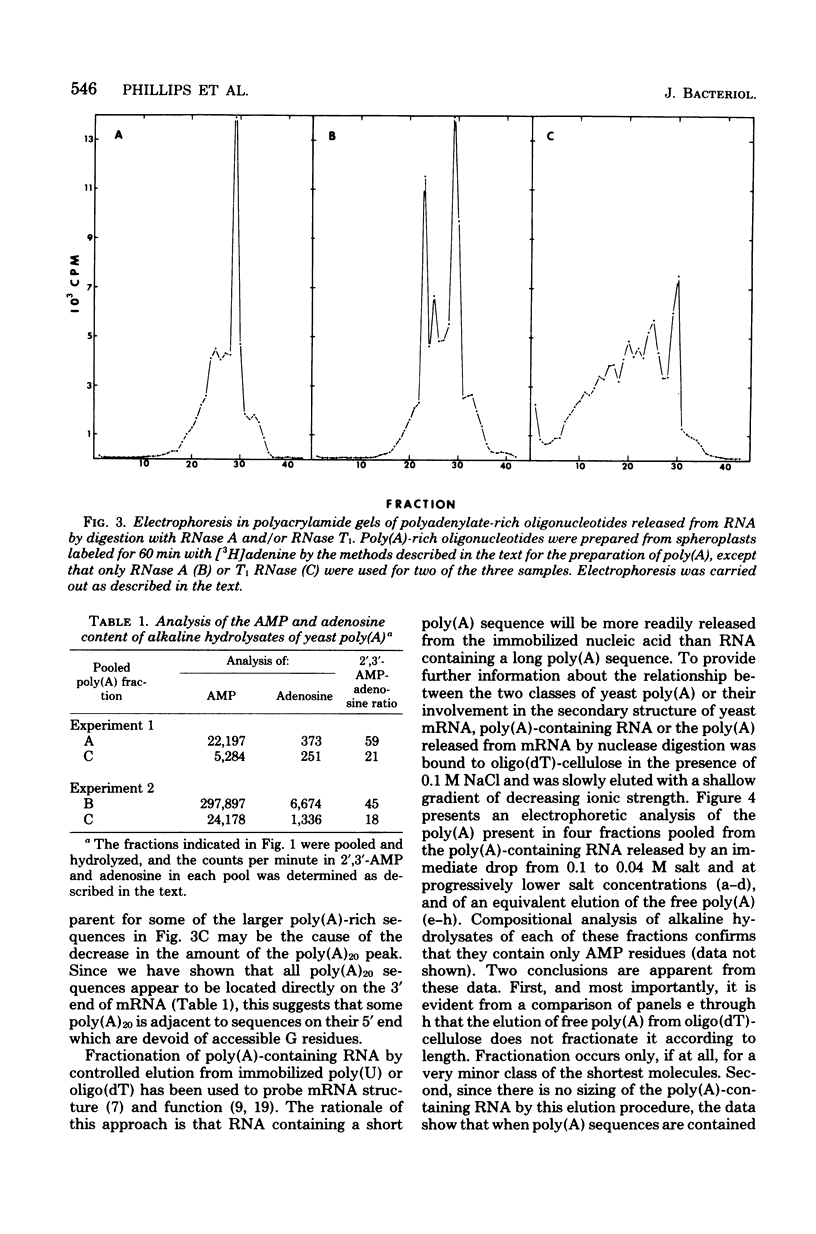
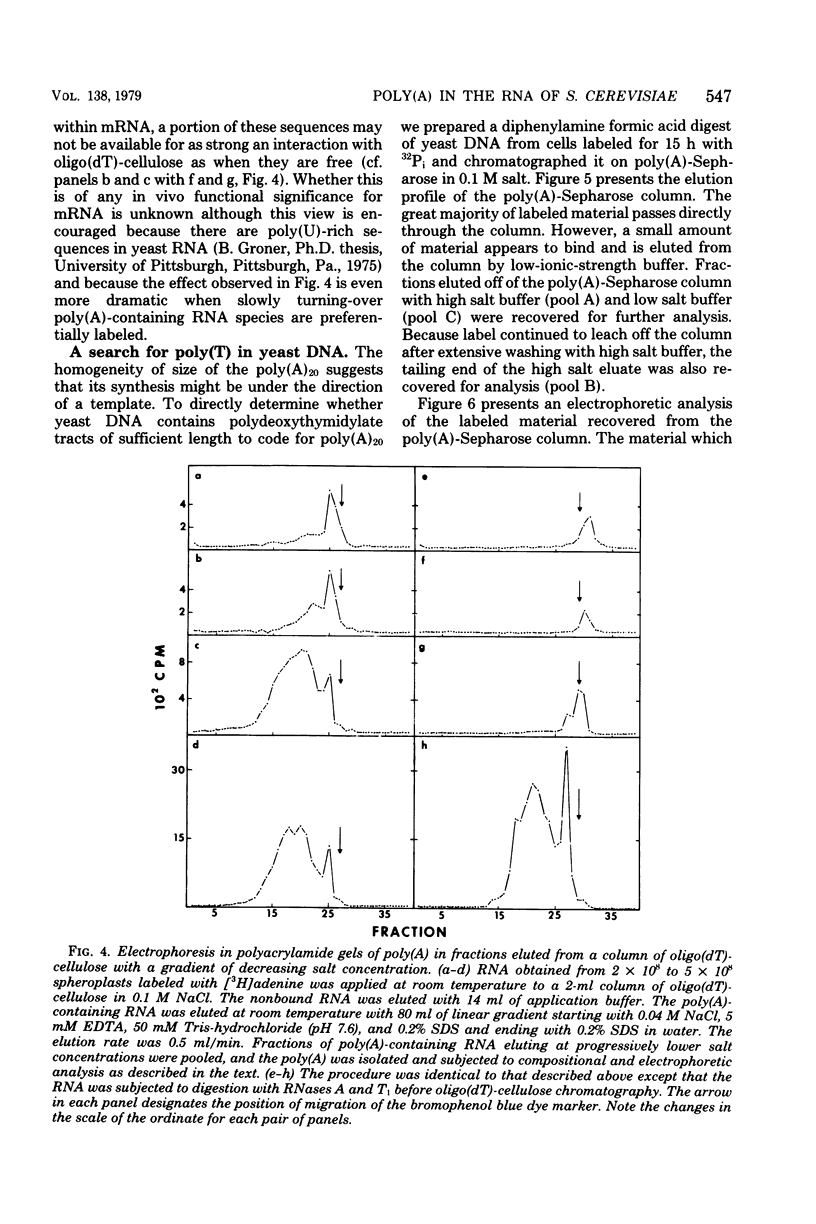
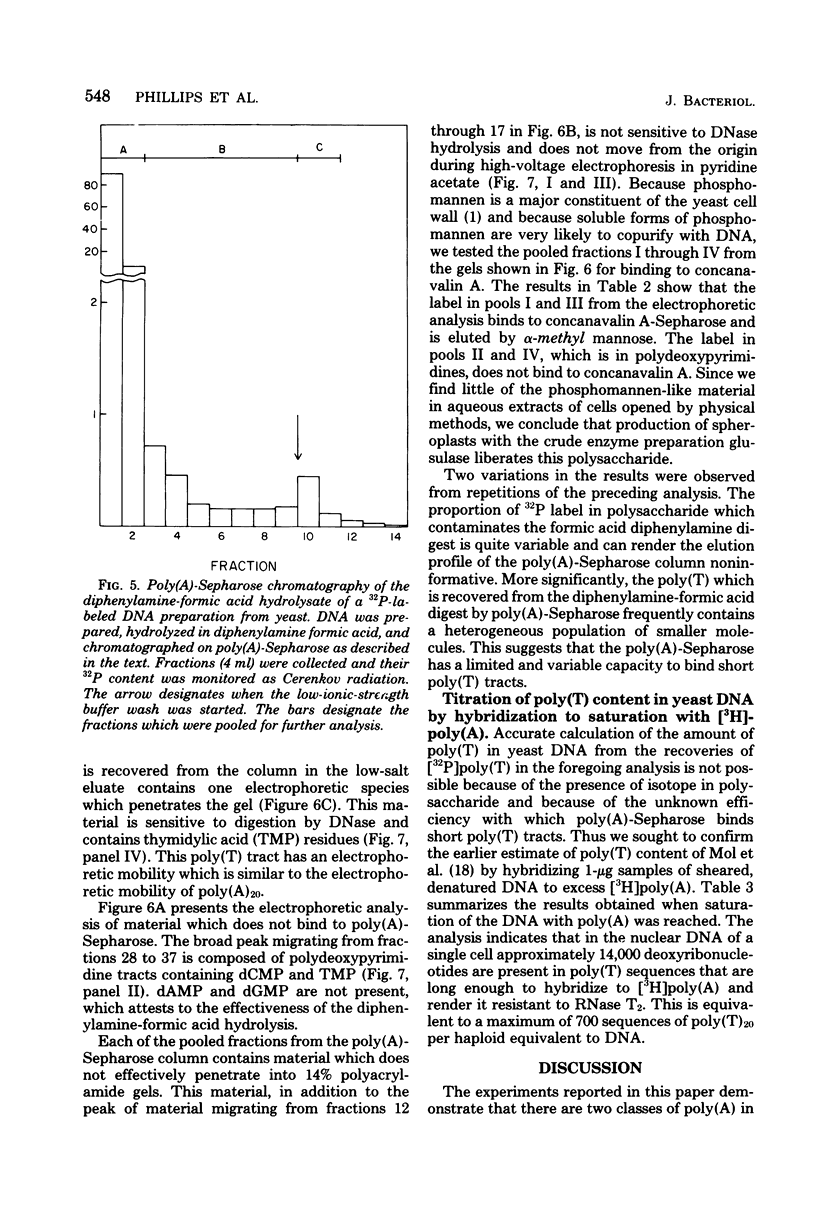
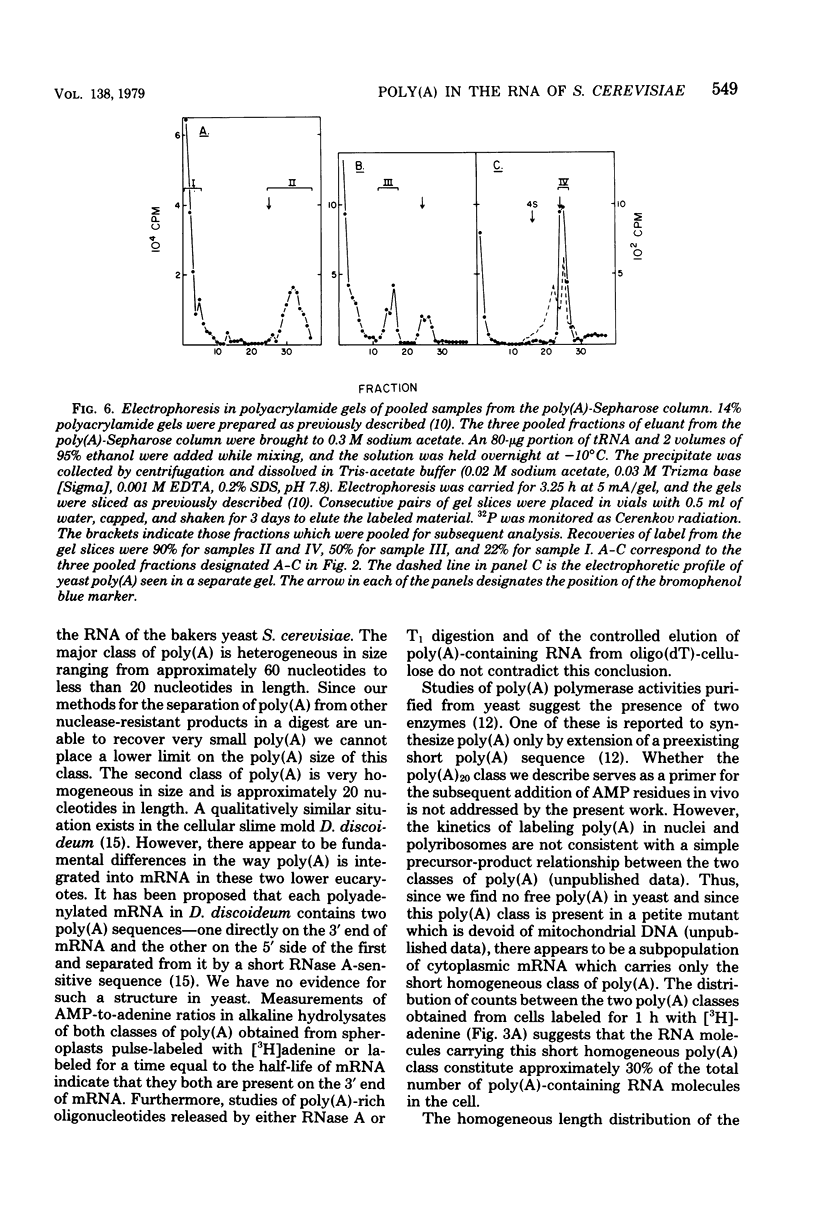
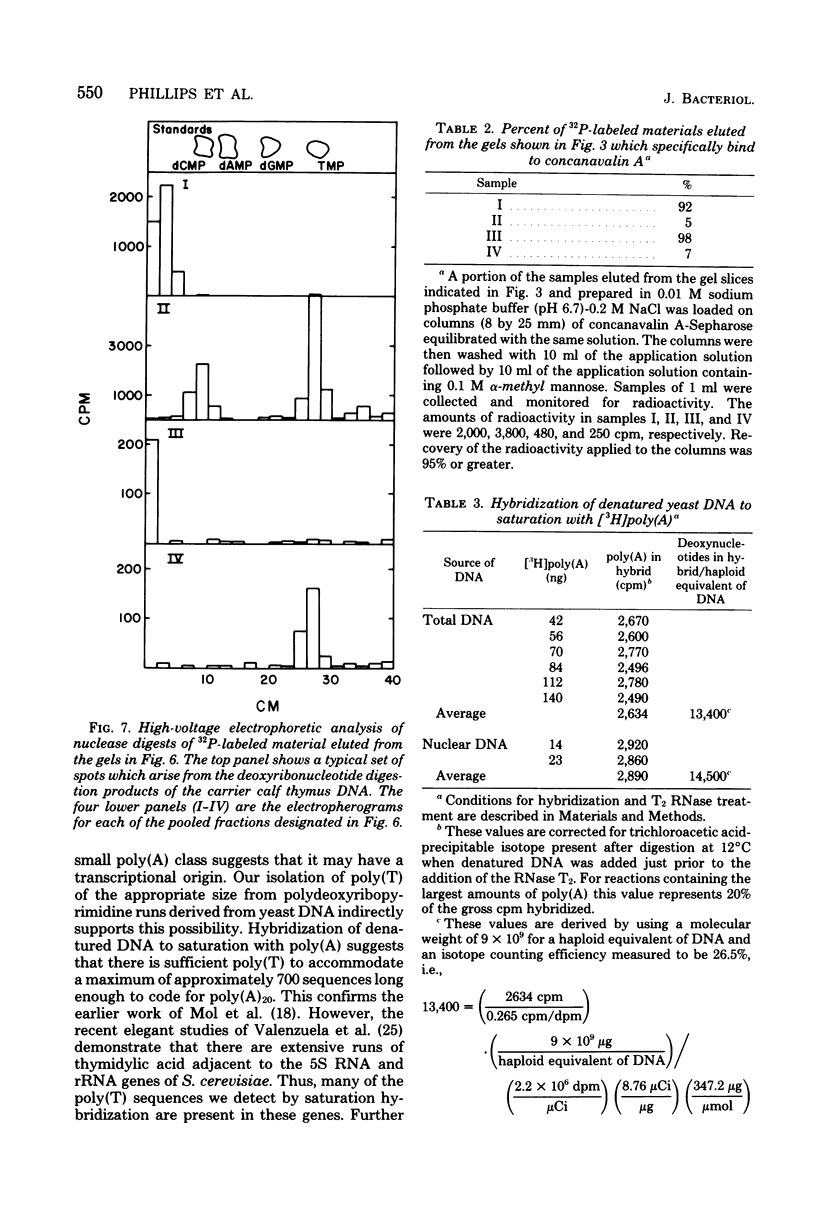
Investigations of the structure of polyadenylic acid [poly(A)] in yeast have shown that there are two classes of poly(A) distinguished by size and kinetics of synthesis. Each class is found directly on the 3' end of messenger RNA. One class contains poly(A) molecules ranging from 60 to less than 20 nucleotides long. The longest molecules in this poly(A) class are the first to become labeled when cells are exposed to [3H]adenine. Label then appears in progressively smaller molecules. The second class of poly(A) is about 20 nucleotides long. The length homogeneity of this class and the presence in nuclear DNA of many copies of a polythymidylate sequence which is the same length suggests that this poly(A) is synthesized by transcription from DNA.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballou C. Structure and biosynthesis of the mannan component of the yeast cell envelope. Adv Microb Physiol. 1976;14(11):93–158. doi: 10.1016/s0065-2911(08)60227-1. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Mitchel R. E., Straus N. A. Analysis of long pyrimidine polynucleotides in HeLa cell nuclear DNA: absence of polydeoxythymidylate. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2189–2192. doi: 10.1073/pnas.70.8.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawerman G. Characteristics and significance of the polyadenylate sequence in mammalian messenger RNA. Prog Nucleic Acid Res Mol Biol. 1976;17:117–148. doi: 10.1016/s0079-6603(08)60068-9. [DOI] [PubMed] [Google Scholar]
- Edmonds M., Caramela M. G. The isolation and characterization of adenosine monophosphate-rich polynucleotides synthesized by Ehrlich ascites cells. J Biol Chem. 1969 Mar 10;244(5):1314–1324. [PubMed] [Google Scholar]
- Edmonds M., Winters M. A. Polyadenylate polymerases. Prog Nucleic Acid Res Mol Biol. 1976;17:149–179. doi: 10.1016/s0079-6603(08)60069-0. [DOI] [PubMed] [Google Scholar]
- Firtel R. A., Kindle K., Huxley M. P. Structural organization and processing of the genetic transcript in the cellular slime mold Dictyostelium discoideum. Fed Proc. 1976 Jan;35(1):13–22. [PubMed] [Google Scholar]
- Forte M. A., Fangman W. L. Naturally occurring cross-links in yeast chromosomal DNA. Cell. 1976 Jul;8(3):425–431. doi: 10.1016/0092-8674(76)90155-0. [DOI] [PubMed] [Google Scholar]
- Gielen J., Aviv H., Leder P. Characteristics of rabbit globin mRNA purification by oligo(dT) cellulose chromatography. Arch Biochem Biophys. 1974 Jul;163(1):146–154. doi: 10.1016/0003-9861(74)90464-0. [DOI] [PubMed] [Google Scholar]
- Groner B., Hynes N., Phillips S. Length heterogeneity in the poly (adenylic acid) region of yeast messenger ribonucleic acid. Biochemistry. 1974 Dec 17;13(26):5378–5383. doi: 10.1021/bi00723a020. [DOI] [PubMed] [Google Scholar]
- Groner B., Phillips S. L. Polyadenylate metabolism in the nuclei and cytoplasm of Saccharomyces cerevisiae. J Biol Chem. 1975 Jul 25;250(14):5640–5646. [PubMed] [Google Scholar]
- Hafe L. A., Keller E. B. The polyadenylate polymerases from yeast. J Biol Chem. 1975 Mar 10;250(5):1838–1846. [PubMed] [Google Scholar]
- Hutchison H. T., Hartwell L. H. Macromolecule synthesis in yeast spheroplasts. J Bacteriol. 1967 Nov;94(5):1697–1705. doi: 10.1128/jb.94.5.1697-1705.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes N. E., Phillips S. L. Turnover of polyadenylate-containing ribonucleic acid in Saccharomyces cerevisiae. J Bacteriol. 1976 Feb;125(2):595–600. doi: 10.1128/jb.125.2.595-600.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson A., Firtel R. A., Lodish H. Transcription of polydeoxythymidylate sequences in the genome of the cellular slime mold, Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1974 May;71(5):1607–1611. doi: 10.1073/pnas.71.5.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R. M., Dyer T. A., Poole C. E., Smith W. The determination of base ratios of very small samples of ribonucleic acid using thin layer chromatography. J Chromatogr. 1968 Apr 23;34(3):364–369. doi: 10.1016/0021-9673(68)80068-8. [DOI] [PubMed] [Google Scholar]
- McLaughlin C. S., Warner J. R., Edmonds M., Nakazato H., Vaughan M. H. Polyadenylic acid sequences in yeast messenger ribonucleic acid. J Biol Chem. 1973 Feb 25;248(4):1466–1471. [PubMed] [Google Scholar]
- Mol J. N., Flavell R. A., Borst P. The presence of (dA.dT)20-25 tracts in the DNA of primitive eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2367–2377. doi: 10.1093/nar/3.9.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison M. R., Gorski J., Lingrel J. B. The separation of mouse reticulocyte 9S RNA into fractions of different biological activity by hybridization to poly(U)-cellulose. Biochem Biophys Res Commun. 1972 Nov 1;49(3):775–781. doi: 10.1016/0006-291x(72)90478-0. [DOI] [PubMed] [Google Scholar]
- Nakazato H., Edmonds M., Kopp D. W. Differential metabolism of large and small poly(A) sequences in the heterogeneous nuclear RNA of HeLa cells. Proc Natl Acad Sci U S A. 1974 Jan;71(1):200–204. doi: 10.1073/pnas.71.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J., Wintersberger E. Adenylic acid-rich sequences in messenger RNA from yeast polysomes. FEBS Lett. 1973 Jun 1;32(2):213–217. doi: 10.1016/0014-5793(73)80835-x. [DOI] [PubMed] [Google Scholar]
- SEBRING E. D., SALZMAN N. P. AN IMPROVED PROCEDURE FOR MEASURING THE DISTRIBUTION OF P32O4--AMONG THE NUCLEOTIDES OF RIBONUCLEIC ACID. Anal Biochem. 1964 May;8:126–129. doi: 10.1016/0003-2697(64)90177-0. [DOI] [PubMed] [Google Scholar]
- Valenzuela P., Bell G. I., Venegas A., Sewell E. T., Masiarz F. R., DeGennaro L. J., Weinberg F., Rutter W. J. Ribosomal RNA genes of Saccharomyces cerevisiae. II. Physical map and nucleotide sequence of the 5 S ribosomal RNA gene and adjacent intergenic regions. J Biol Chem. 1977 Nov 25;252(22):8126–8135. [PubMed] [Google Scholar]



