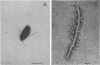
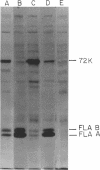
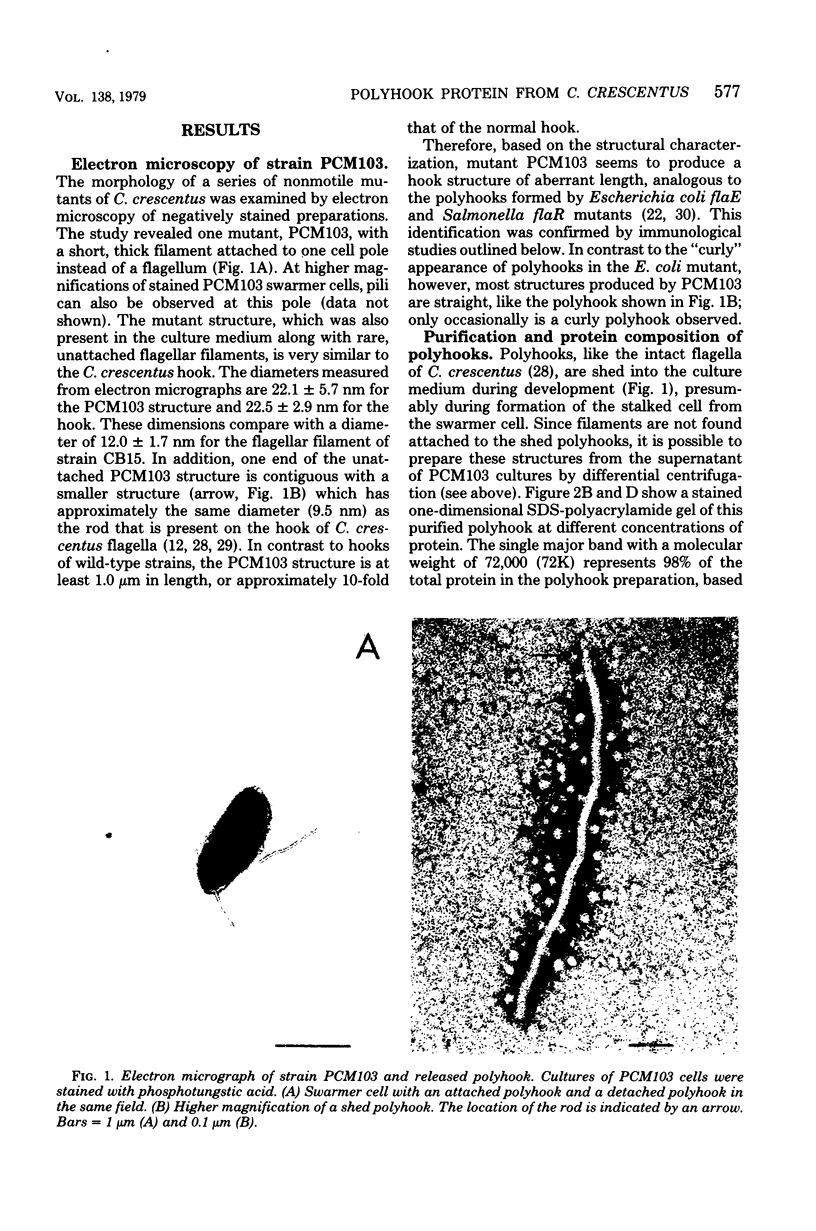
Abstract
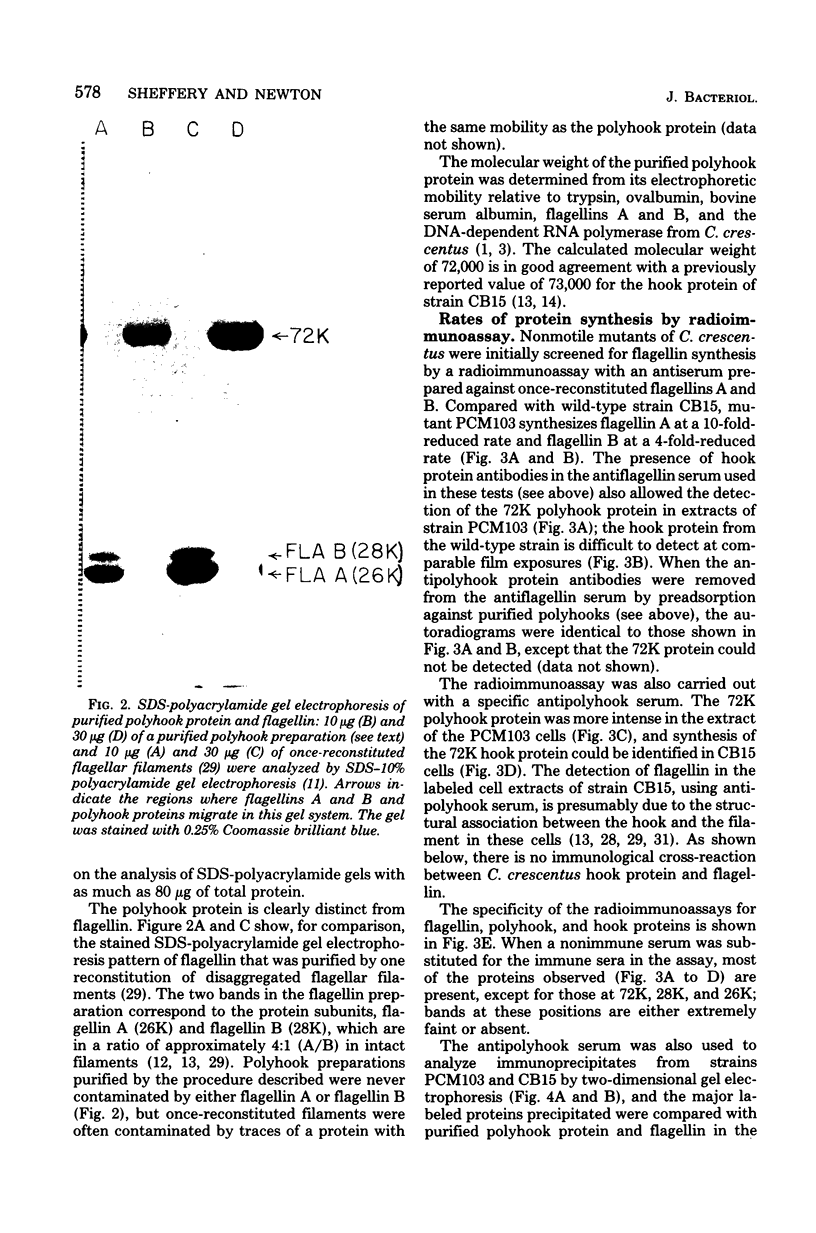
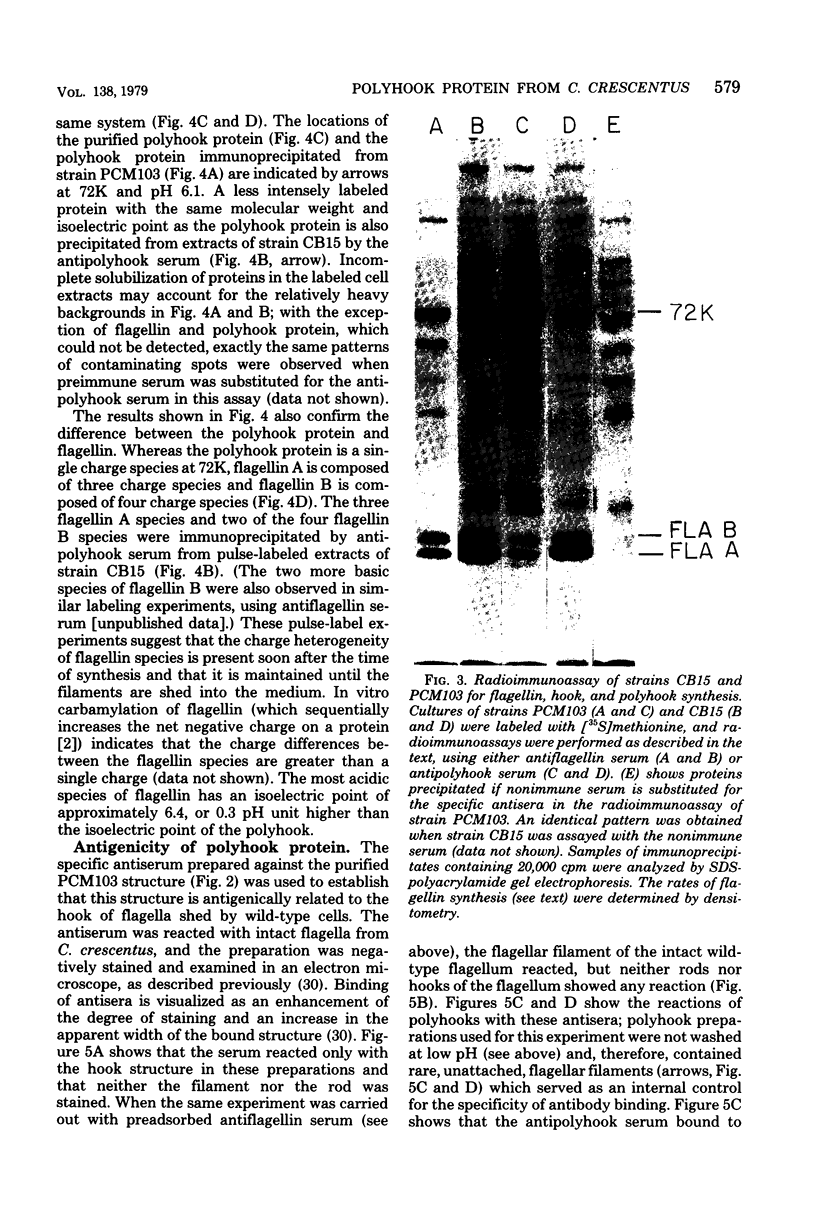
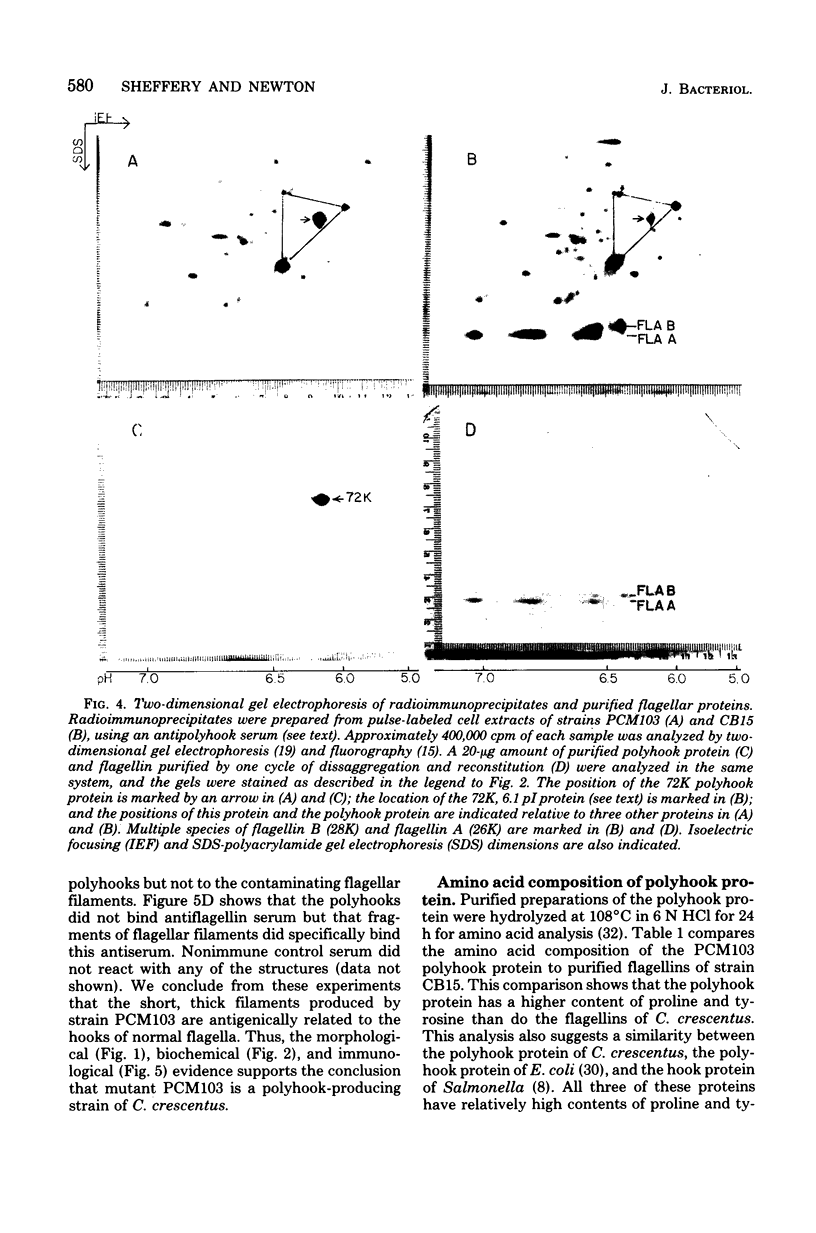
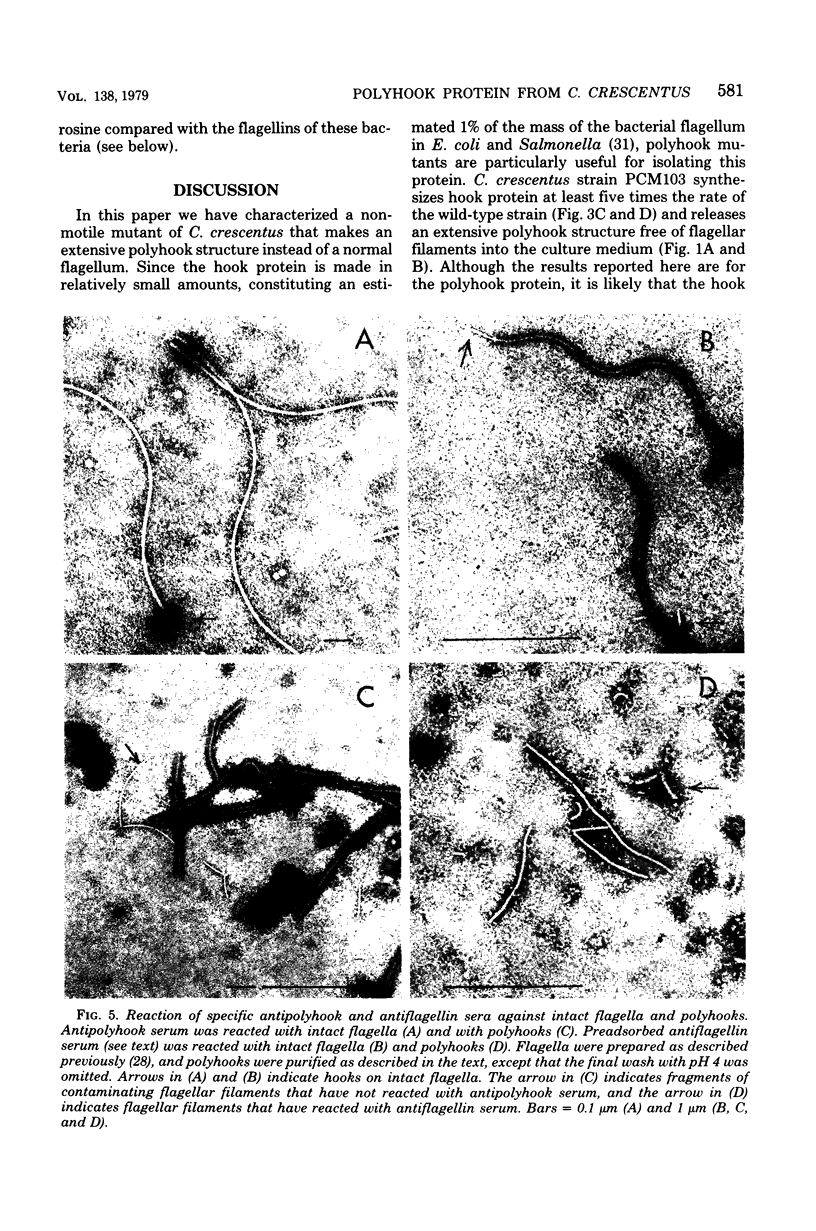
A polyhook-producing strain of Caulobacter crescentus was isolated, and the polyhook protein was purified. The antigenicity and morphology of the polyhook structure are similar to the wild-type hook except that the mutant strain produces a hook structure at least 10-fold the length of wild-type hooks (1.0 versus 0.1 micrometers). The molecular weight of the polyhook protein, as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, is 72,000, and the protein has a pI of approximately 6.1. Antibodies prepared against the polyhook protein were used to show that this protein is antigenically distinct from the Caulobacter flagellins. Amino acid analysis of the polyhook protein revealed compositional similarities to other gram-negative, bacterial hook proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amemiya K., Wu C. W., Shapiro L. Caulobacter crescentus RNA polymerase. Purification and characterization of holoenzyme and core polymerase. J Biol Chem. 1977 Jun 25;252(12):4157–4165. [PubMed] [Google Scholar]
- Bobb D., Hofstee B. H. Gel isoelectric focusing for following the successive carbamylations of amino groups in chymotrypsinogen A. Anal Biochem. 1971 Mar;40(1):209–217. doi: 10.1016/0003-2697(71)90094-7. [DOI] [PubMed] [Google Scholar]
- Cheung K. K., Newton A. Polyadenylic acid synthesis activity of purified DNA-dependent RNA polymerase from Caulobacter. J Biol Chem. 1978 Apr 10;253(7):2254–2261. [PubMed] [Google Scholar]
- Fukuda A., Miyakawa K., Iida H., Okada Y. Regulation of polar surface structures in Caulobacter crescentus: pleiotropic mutations affect the coordinate morphogenesis of flagella, pili and phage receptors. Mol Gen Genet. 1976 Dec 8;149(2):167–173. doi: 10.1007/BF00332885. [DOI] [PubMed] [Google Scholar]
- Iino T. Genetics of structure and function of bacterial flagella. Annu Rev Genet. 1977;11:161–182. doi: 10.1146/annurev.ge.11.120177.001113. [DOI] [PubMed] [Google Scholar]
- Kagawa H., Asakura S., Iino T. Serological study of bacterial flagellar hooks. J Bacteriol. 1973 Mar;113(3):1474–1481. doi: 10.1128/jb.113.3.1474-1481.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa H., Owaribe K., Asakura S., Takahashi N. Flagellar hook protein from Salmonella SJ25. J Bacteriol. 1976 Jan;125(1):68–73. doi: 10.1128/jb.125.1.68-73.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kurn N., Ammer S., Shapiro L. A pleiotropic mutation affecting expression of polar development events in Caulobacter crescentus. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3157–3161. doi: 10.1073/pnas.71.8.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lagenaur C., Agabian N. Caulobacter flagellar organelle: synthesis, compartmentation, and assembly. J Bacteriol. 1978 Sep;135(3):1062–1069. doi: 10.1128/jb.135.3.1062-1069.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagenaur C., Agabian N. Physical characterization of Caulobacter crescentus flagella. J Bacteriol. 1976 Oct;128(1):435–444. doi: 10.1128/jb.128.1.435-444.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagenaur C., DeMartini M., Agabian N. Isolation and characterization of Caulobacter crescentus flagellar hooks. J Bacteriol. 1978 Nov;136(2):795–798. doi: 10.1128/jb.136.2.795-798.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Marino W., Ammer S., Shapiro L. Conditional surface structure mutants of Caulobacter crescentus temperature-sensitive flagella formation due to an altered flagellin monomer. J Mol Biol. 1976 Oct 25;107(2):115–130. doi: 10.1016/s0022-2836(76)80021-6. [DOI] [PubMed] [Google Scholar]
- Metzger H., Shapiro M. B., Mosimann J. E., Vinton J. E. Assessment of compositional relatedness between proteins. Nature. 1968 Sep 14;219(5159):1166–1168. doi: 10.1038/2191166a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Osley M. A., Newton A. Mutational analysis of developmental control in Caulobacter crescentus. Proc Natl Acad Sci U S A. 1977 Jan;74(1):124–128. doi: 10.1073/pnas.74.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osley M. A., Sheffery M., Newton A. Regulation of flagellin synthesis in the cell cycle of caulobacter: dependence on DNA replication. Cell. 1977 Oct;12(2):393–400. doi: 10.1016/0092-8674(77)90115-5. [DOI] [PubMed] [Google Scholar]
- POINDEXTER J. S. BIOLOGICAL PROPERTIES AND CLASSIFICATION OF THE CAULOBACTER GROUP. Bacteriol Rev. 1964 Sep;28:231–295. doi: 10.1128/br.28.3.231-295.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson-Delafield J., Martinez R. J., Stocker B. A., Yamaguchi S. A new fla gene in Salmonella typhimurium--flaR--and its mutant phenotype-superhooks. Arch Mikrobiol. 1973 Mar 26;90(2):107–120. doi: 10.1007/BF00414513. [DOI] [PubMed] [Google Scholar]
- Poindexter J. S., Hornack P. R., Armstrong P. A. Intracellular development of a large DNA bacteriophage lytic for Caulobacter crescentus. Arch Mikrobiol. 1967;59(1):237–246. doi: 10.1007/BF00406337. [DOI] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W. Proteolytic cleavage of bacteriophage lambda repressor in induction. Proc Natl Acad Sci U S A. 1975 Jan;72(1):147–151. doi: 10.1073/pnas.72.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. M., Stanier R. Y. The development of cellular stalks in bacteria. J Cell Biol. 1966 Mar;28(3):423–436. doi: 10.1083/jcb.28.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L., Agabian-Keshishian N., Bendis I. Bacterial differentiation. Science. 1971 Sep 3;173(4000):884–892. doi: 10.1126/science.173.4000.884. [DOI] [PubMed] [Google Scholar]
- Shapiro L., Maizel J. V., Jr Synthesis and structure of Caulobacter crescentus flagella. J Bacteriol. 1973 Jan;113(1):478–485. doi: 10.1128/jb.113.1.478-485.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffery M., Newton A. Reconstitution and purification of flagellar filaments from Caulobacter crescentus. J Bacteriol. 1977 Dec;132(3):1027–1030. doi: 10.1128/jb.132.3.1027-1030.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M. R., Simon M. I. Flagellar assembly mutants in Escherichia coli. J Bacteriol. 1972 Nov;112(2):986–993. doi: 10.1128/jb.112.2.986-993.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M., Simon M. I. Bacterial flagella. Annu Rev Microbiol. 1977;31:397–419. doi: 10.1146/annurev.mi.31.100177.002145. [DOI] [PubMed] [Google Scholar]