Abstract
About half of pneumococci recovered from pediatric patients and one-third of isolates from adult patients yielded bacteriophages active against one or more of four noncapsulated indicator strains of pneumococcus. Strains of capsular types most frequently causing pediatric infections were associated with lysogeny. Classical restriction-modification phenomena have been demonstrated in vivo with some of the temperate phages, and correlation of restriction with the presence of one or the other of the two known pneumococcal restriction endonucleases has been established. The temperate phages differ serologically and in several other characteristics from virulent pneumococcal phages previously described. All pneumococcal phages so far studied can be classified into a minimum of three serological groups.
Full text
PDF

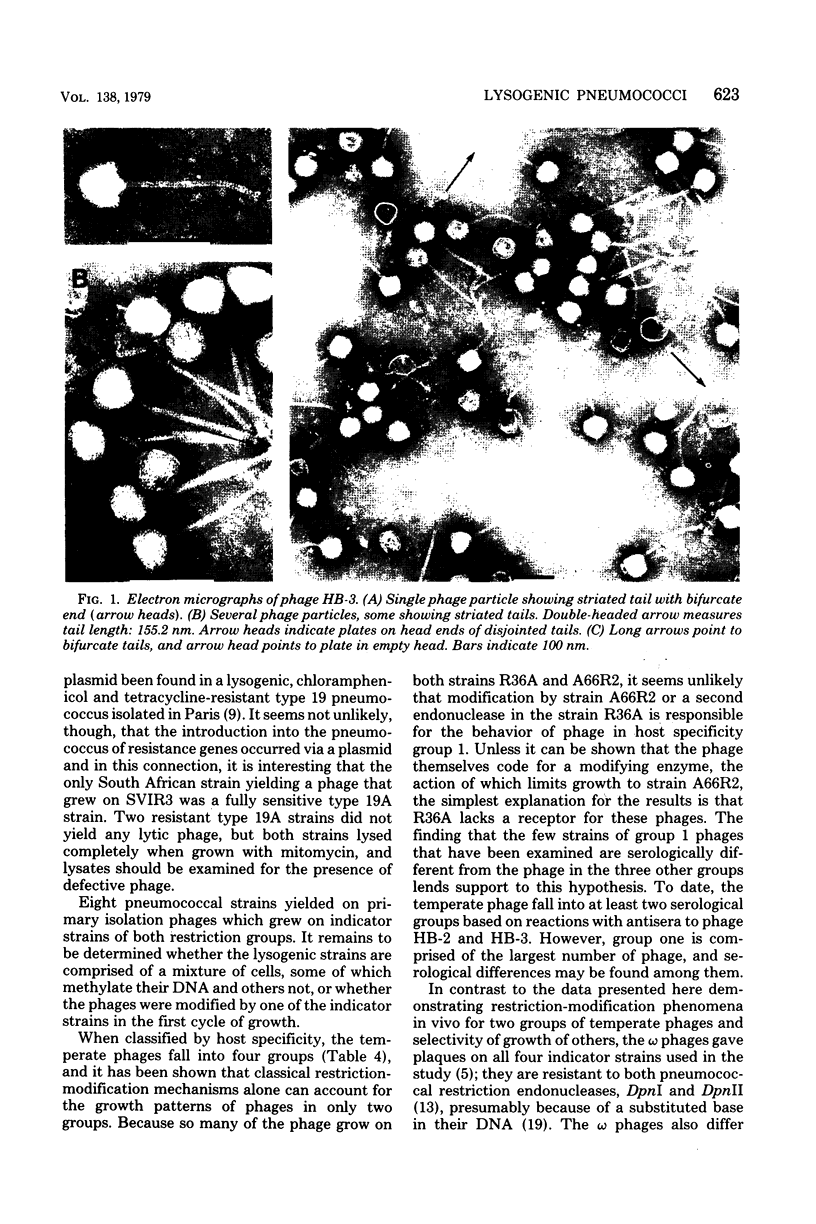

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUSTRIAN R., BERNHEIMER H. P., SMITH E. E., MILLS G. T. Simultaneous production of two capsular polysaccharides by pneumococcus. II. The genetic and biochemical bases of binary capsulation. J Exp Med. 1959 Oct 1;110:585–602. doi: 10.1084/jem.110.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer H. P. Lysogeny in pneumococci freshly isolated from man. Science. 1977 Jan 7;195(4273):66–68. doi: 10.1126/science.12565. [DOI] [PubMed] [Google Scholar]
- Bernheimer H. P., Tiraby J. G. Inhibition of phage infection by pneumococcus capsule. Virology. 1976 Aug;73(1):308–309. doi: 10.1016/0042-6822(76)90085-4. [DOI] [PubMed] [Google Scholar]
- Bernheimer H. P., Wermundsen I. E., Austrian R. Qualitative differences in the behavior of pneumoncoccal deoxyribonucleic acids transforming to the same capsular type. J Bacteriol. 1967 Jan;93(1):320–333. doi: 10.1128/jb.93.1.320-333.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. M., Calandra G. B., Huff E., Nugent K. M. Attributes of potential utility in differentiating among "group H" streptococci or Streptococcus sanguis. J Dent Res. 1976 Jan;55:A142–A153. doi: 10.1177/002203457605500106011. [DOI] [PubMed] [Google Scholar]
- Dang-Van A., Tiraby G., Acar J. F., Shaw W. V., Bouanchaud D. H. Chloramphenicol resistance in Streptococcus pneumoniae: enzymatic acetylation and possible plasmid linkage. Antimicrob Agents Chemother. 1978 Apr;13(4):577–583. doi: 10.1128/aac.13.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACKS S., HOTCHKISS R. D. A study of the genetic material determining an enzyme in Pneumococcus. Biochim Biophys Acta. 1960 Apr 22;39:508–518. doi: 10.1016/0006-3002(60)90205-5. [DOI] [PubMed] [Google Scholar]
- Lacks S., Greenberg B. A deoxyribonuclease of Diplococcus pneumoniae specific for methylated DNA. J Biol Chem. 1975 Jun 10;250(11):4060–4066. [PubMed] [Google Scholar]
- Lopez R., Ronda C., Tomasz A., Portoles A. Properties of "diplophage": a lipid-containing bacteriophage. J Virol. 1977 Oct;24(1):201–210. doi: 10.1128/jvi.24.1.201-210.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACLEOD C. M., KRAUSS M. R. Transformation reactions with two non-allelic R mutants of the same strain of Pneumococcus type VIII. J Exp Med. 1956 May 1;103(5):623–632. doi: 10.1084/jem.103.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcdonnell M., Lain R., Tomasz A. "Diplophage": a bacteriophage of Diplococcus pneumoniae. Virology. 1975 Feb;63(2):577–582. doi: 10.1016/0042-6822(75)90329-3. [DOI] [PubMed] [Google Scholar]
- Porter R. D., Guild W. R. Characterization of some pneumococcal bacteriophages. J Virol. 1976 Aug;19(2):659–667. doi: 10.1128/jvi.19.2.659-667.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R. D., Shoemaker N. B., Rampe G., Guild W. R. Bacteriophage-associated gene transfer in pneumococcus: transduction or pseudotransduction? J Bacteriol. 1979 Jan;137(1):556–567. doi: 10.1128/jb.137.1.556-567.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosan B. Antigens of Streptococcus sanguis. Infect Immun. 1973 Feb;7(2):205–211. doi: 10.1128/iai.7.2.205-211.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiraby J. G., Tiraby E., Fox M. S. Pneumococcal bacteriophages. Virology. 1975 Dec;68(2):566–569. doi: 10.1016/0042-6822(75)90300-1. [DOI] [PubMed] [Google Scholar]
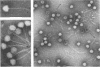
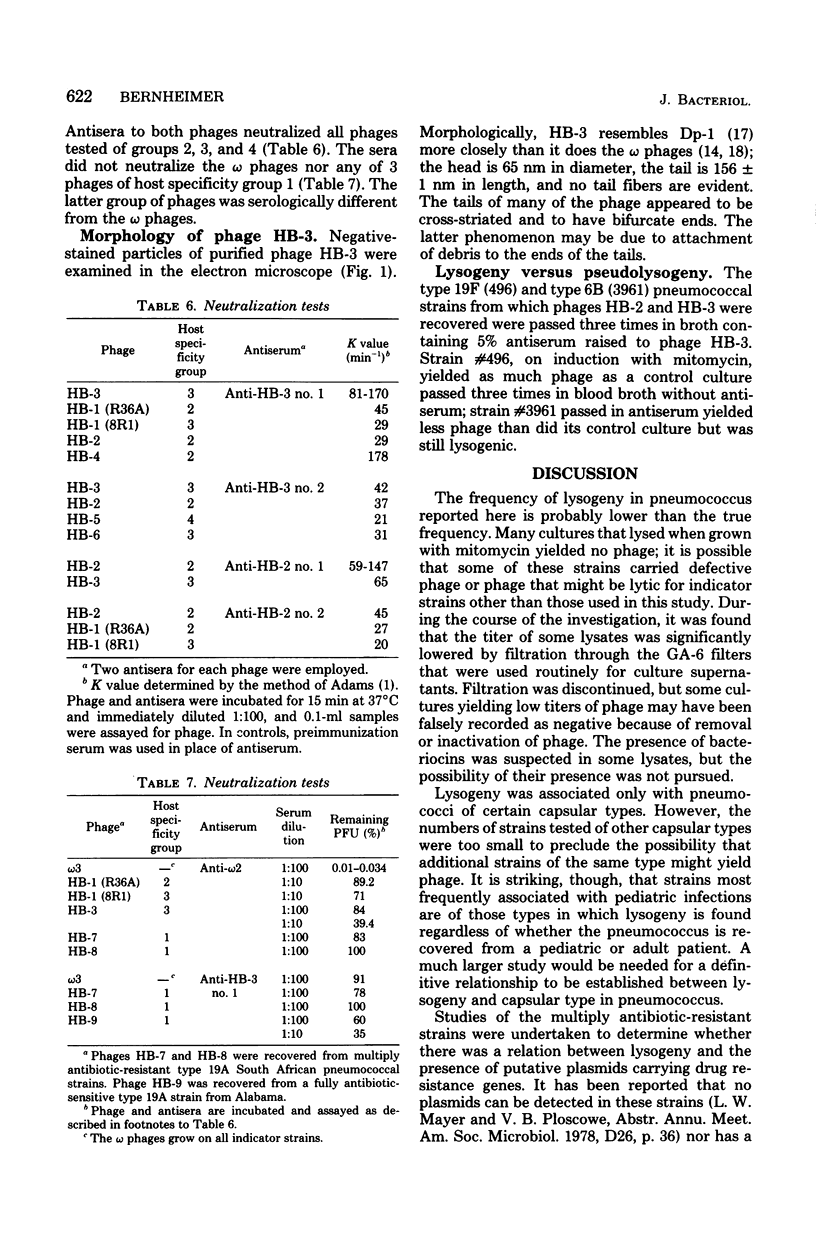
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]