Abstract
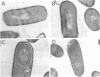
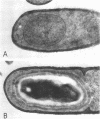
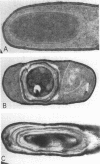
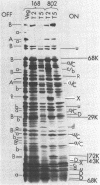
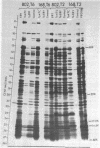
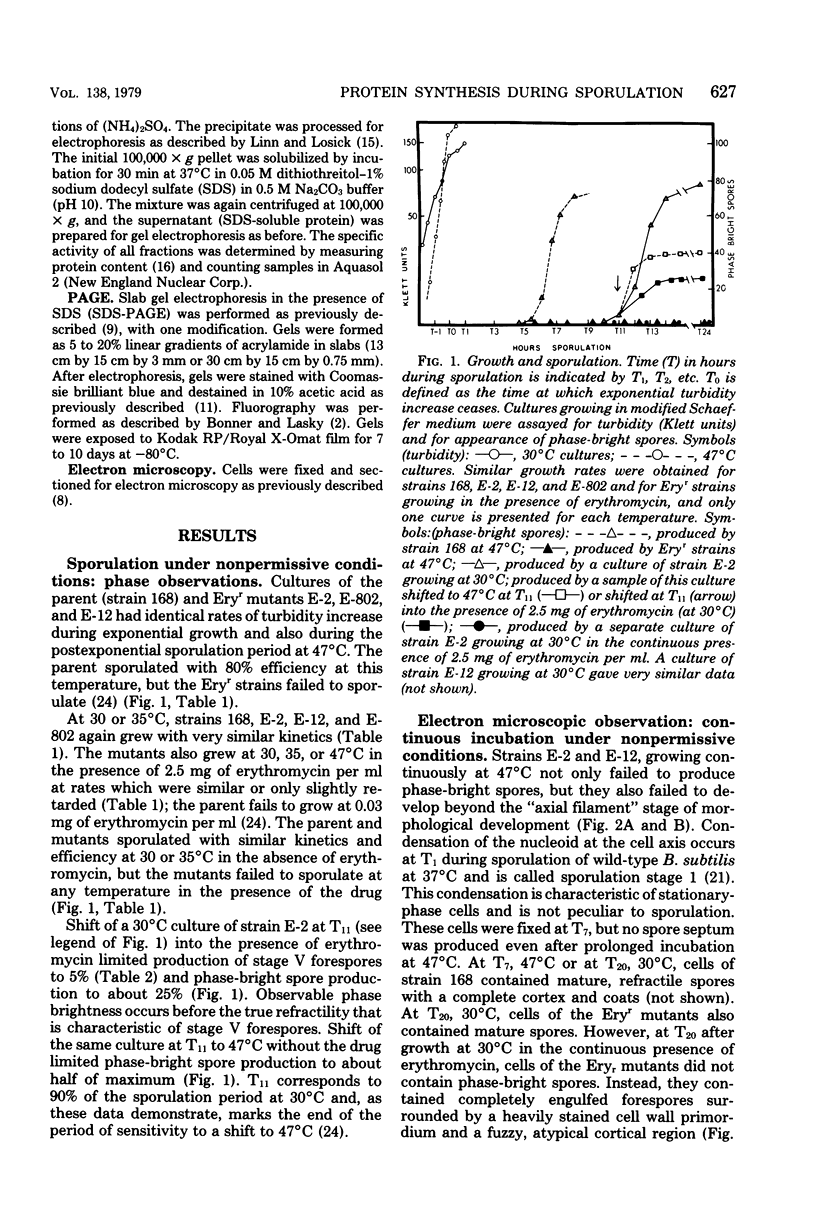
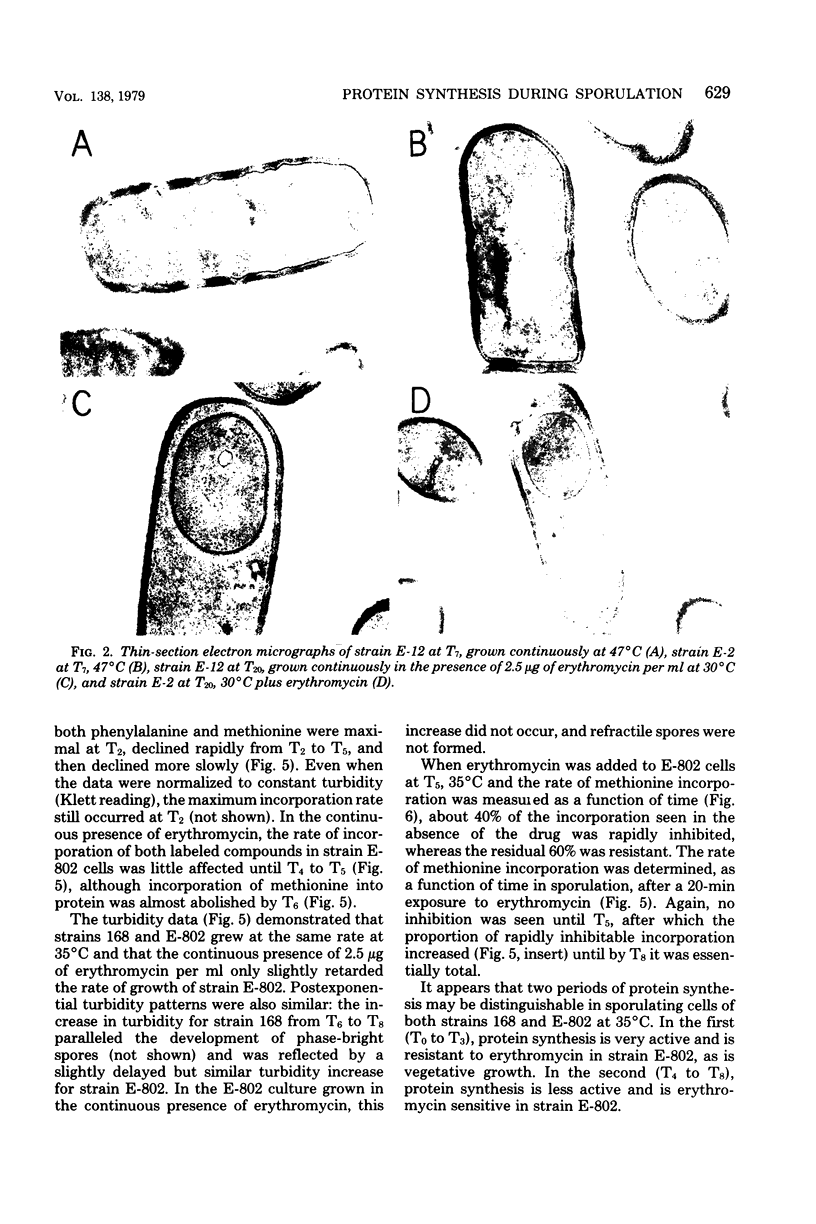
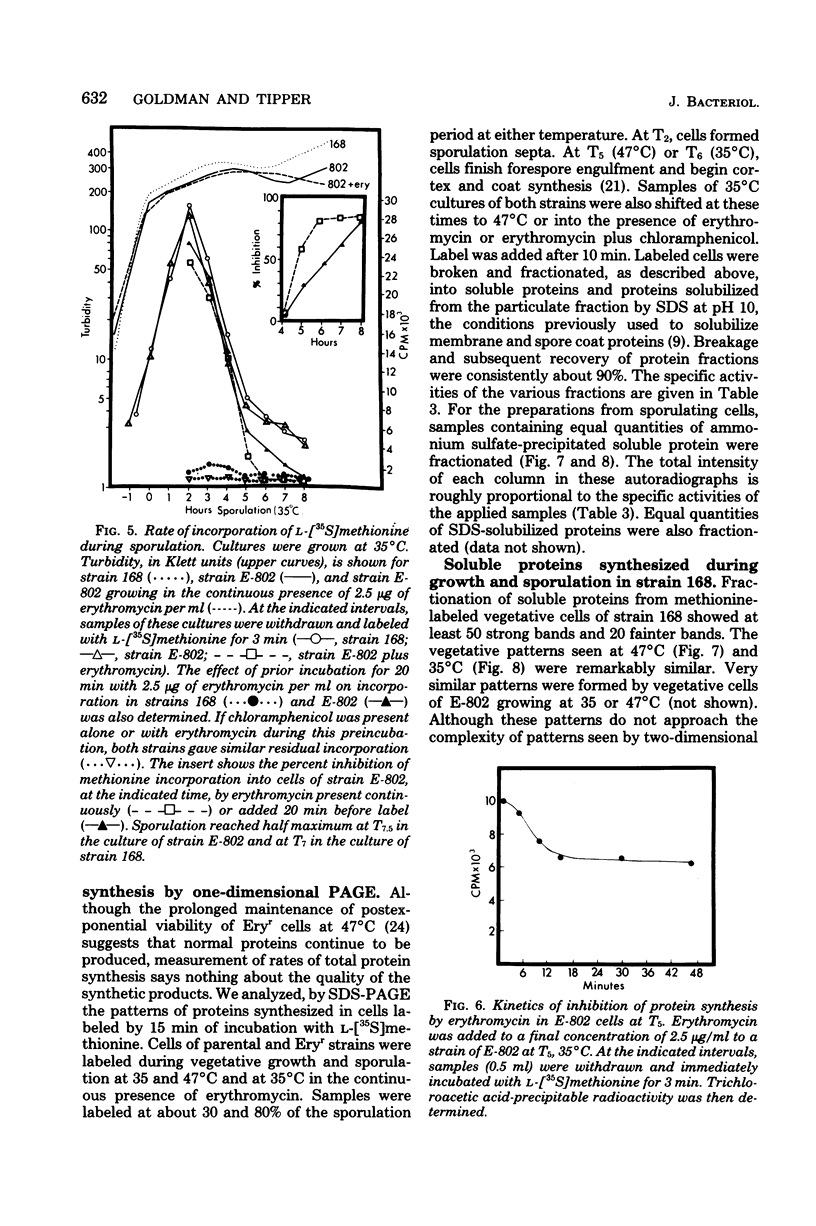
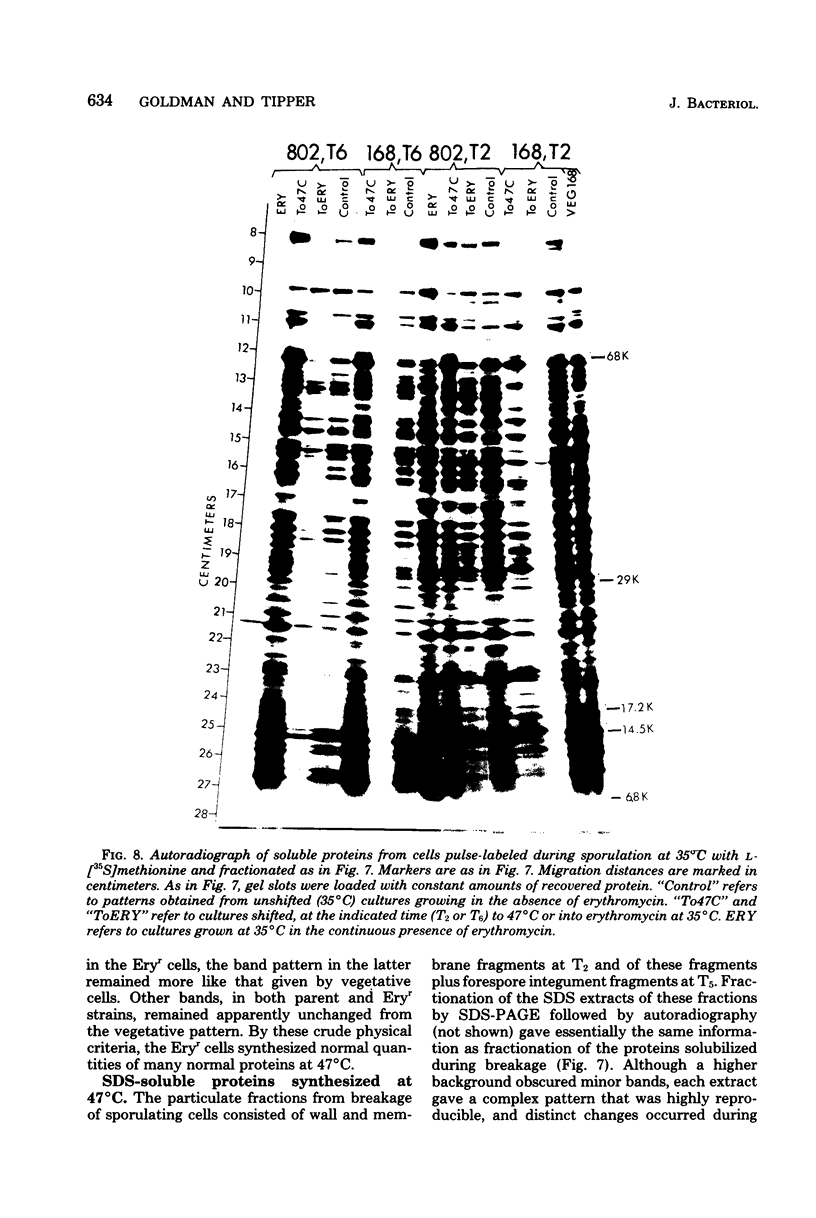
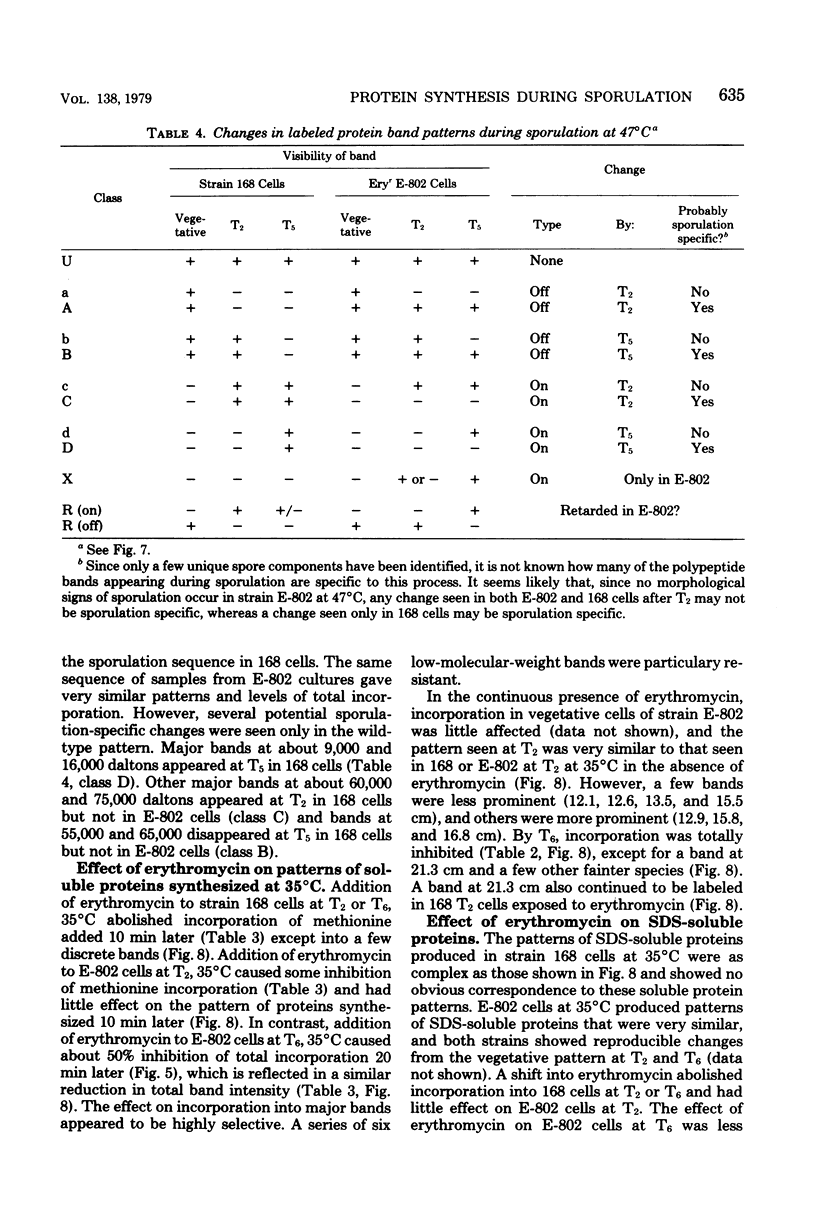
Erythromycin-resistant (Eryr) mutants of Bacillus subtilis 168 fail to sporulate at high temperature (47 degrees C) but sporulate normally at 30 to 35 degrees C. They also fail to sporulate at any temperature in the presence of 2.5 micrograms of erythromycin per ml. Neither of these nonpermissive conditions appears to affect vegetative growth, and the periods of sensitivity to both conditions extend from 40 to 90% of the sporulation period. At 47 degrees C, net incorporation of methionine and phenylalanine in postexponential Eryr and 168 cells was similar, and fractionation of the labeled products by polyacrylamide gel electrophoresis gave patterns in which many of the bands produced by mutant and parental cells coincided. However, distinct differences were seen, and since no spore-specific morphogenesis occurred in the Eryr cells at 47 degrees C, a selective defect in spore gene expression was inferred. At 35 degrees C plus erythromycin, spore morphogenesis proceeded normally until forespores were produced and then ceased, coincident with a marked increase in sensitivity of total protein synthesis to erythromycin. The effects seem to be nonspecific, therefore, and may indicate a change in cell permeability or ribosomal sensitivity to erythromycin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chambliss G. H., Legault-Demare L. Functional modifications of the translational system in Bacillus subtilis during sporulation. J Bacteriol. 1977 Oct;132(1):13–22. doi: 10.1128/jb.132.1.13-22.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi R. H. Role of ribonucleic acid polymerase in gene selection in procaryotes. Bacteriol Rev. 1977 Sep;41(3):568–594. doi: 10.1128/br.41.3.568-594.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda R., Doi R. H. Two polypeptides associated with the ribonucleic acid polymerase core of Bacillus subtilis during sporulation. J Bacteriol. 1977 Jan;129(1):422–432. doi: 10.1128/jb.129.1.422-432.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. C. Membrane protein alterations during the early stages of sporulation of Bacillus subtilis. J Supramol Struct. 1976;5(4):457–473. doi: 10.1002/jss.400050405. [DOI] [PubMed] [Google Scholar]
- Goldman R. C., Tipper D. J. Bacillus subtilis spore coats: complexity and purification of a unique polypeptide component. J Bacteriol. 1978 Sep;135(3):1091–1106. doi: 10.1128/jb.135.3.1091-1106.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham R. S., Bott K. F. Antibiotic-resistant mutants of Bacillus subtilis conditional for sporulation. Mol Gen Genet. 1975;137(3):227–237. doi: 10.1007/BF00333018. [DOI] [PubMed] [Google Scholar]
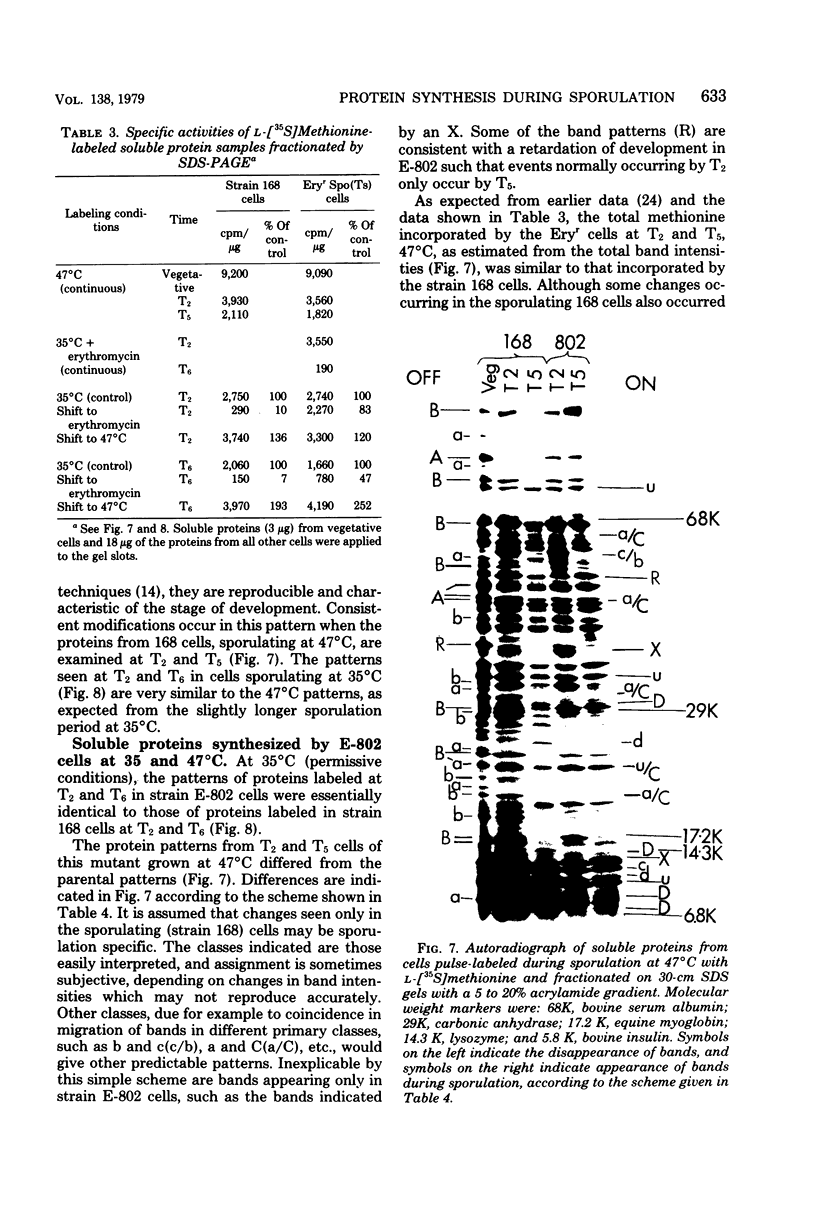
- Guha S., Szulmajster J. Specific alteration of the 30S ribosomal subunits of Bacillus subtilis during sporulation. J Bacteriol. 1977 Sep;131(3):866–871. doi: 10.1128/jb.131.3.866-871.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leighton T. J. An RNA polymerase mutation causing temperature-sensitive sporulation in Bacillus subtilis. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1179–1183. doi: 10.1073/pnas.70.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton T. J., Doi R. H. The stability of messenger ribonucleic acid during sporulation in Bacillus subtilis. J Biol Chem. 1971 May 25;246(10):3189–3195. [PubMed] [Google Scholar]
- Leighton T. Sporulation-specific translational discrimination in Bacillus subtilis. J Mol Biol. 1974 Jul 15;86(4):855–863. doi: 10.1016/0022-2836(74)90358-1. [DOI] [PubMed] [Google Scholar]
- Linn T., Greenleaf A. L., Losick R. RNA polymerase from sporulating Bacillus subtilis. Purification and properties of a modified form of the enzyme containing two sporulation polypeptides. J Biol Chem. 1975 Dec 25;250(24):9256–9261. [PubMed] [Google Scholar]
- Linn T., Losick R. The program of protein synthesis during sporulation in Bacillus subtilis. Cell. 1976 May;8(1):103–114. doi: 10.1016/0092-8674(76)90191-4. [DOI] [PubMed] [Google Scholar]
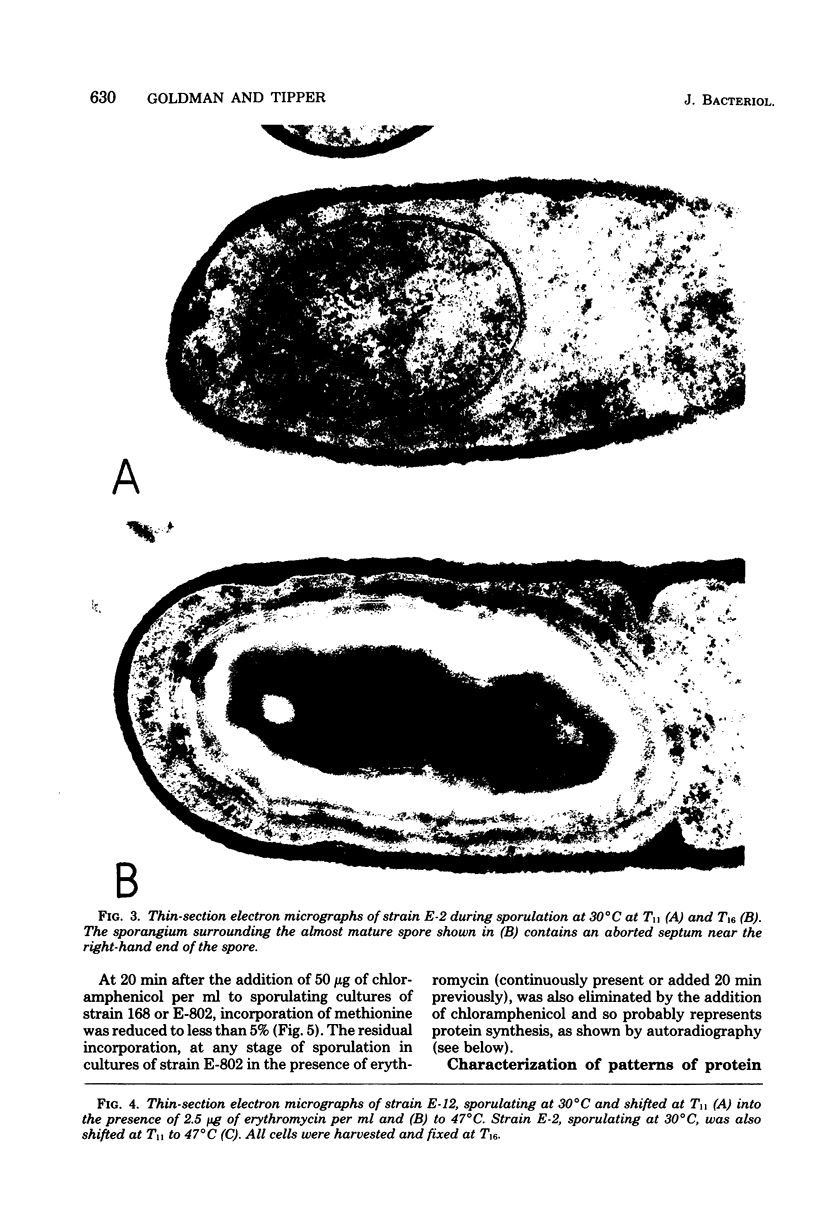
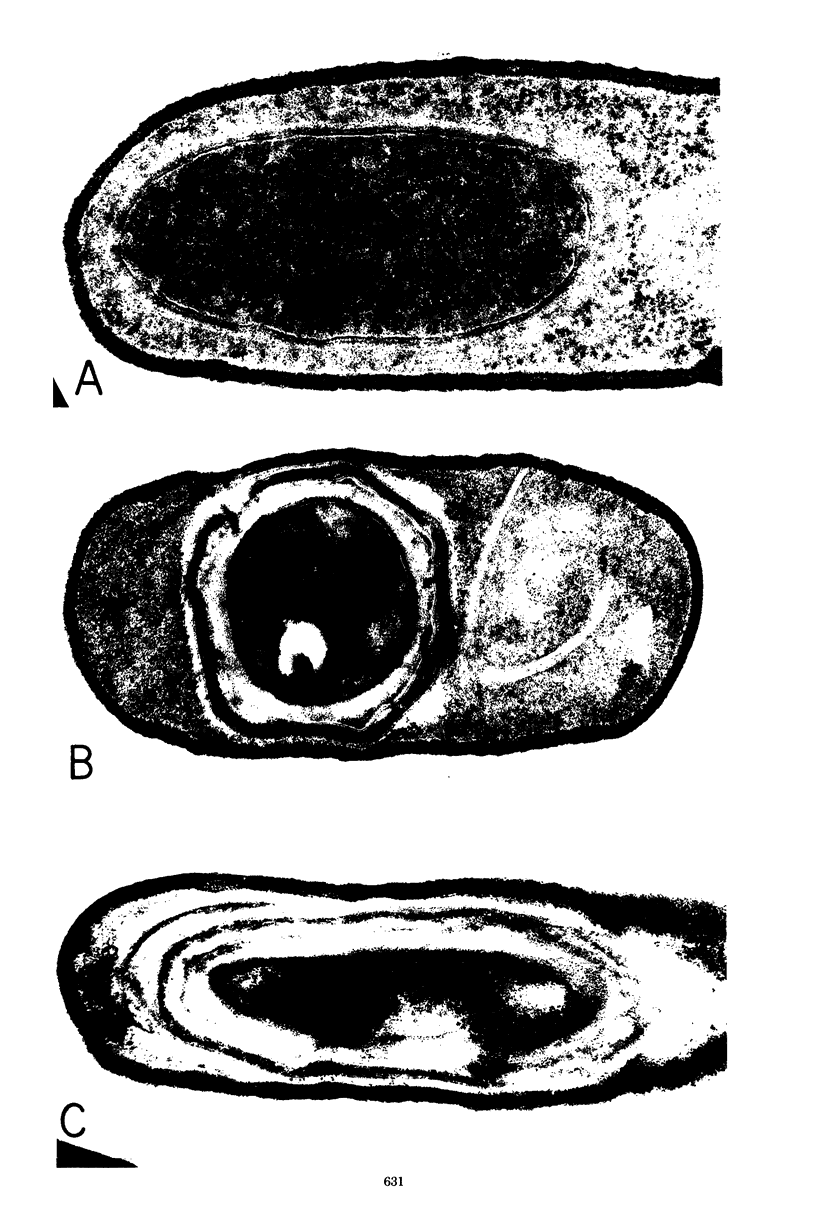
- RYTER A. ETUDE MORPHOLOGIQUE DE LA SPORULATION DE BACILLUS SUBTILIS. Ann Inst Pasteur (Paris) 1965 Jan;108:40–60. [PubMed] [Google Scholar]
- Rhaese H. J., Groscurth R. Control of development: role of regulatory nucleotides synthesized by membranes of Bacillus subtilis in initiation of sporulation. Proc Natl Acad Sci U S A. 1976 Feb;73(2):331–335. doi: 10.1073/pnas.73.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhaese H. J., Hoch J. A., Groscurth R. Studies on the control of development: isolation of Bacillus subtilis mutants blocked early in sporulation and defective in synthesis of highly phosphorylated nucleotides. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1125–1129. doi: 10.1073/pnas.74.3.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa J. C., Silva M. T., Balassa G. An exosporium-like outer layer in Bacillus subtilis spores. Nature. 1976 Sep 2;263(5572):53–54. doi: 10.1038/263053a0. [DOI] [PubMed] [Google Scholar]
- Staal S. P., Hoch J. A. Conditional dihydrostreptomycin resistance in Bacillus subtilis. J Bacteriol. 1972 Apr;110(1):202–207. doi: 10.1128/jb.110.1.202-207.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper D. J., Johnson C. W., Ginther C. L., Leighton T., Wittmann H. G. Erythromycin resistant mutations in Bacillus subtilis cause temperature sensitive sporulation. Mol Gen Genet. 1977 Jan 18;150(2):147–159. doi: 10.1007/BF00695395. [DOI] [PubMed] [Google Scholar]