Abstract
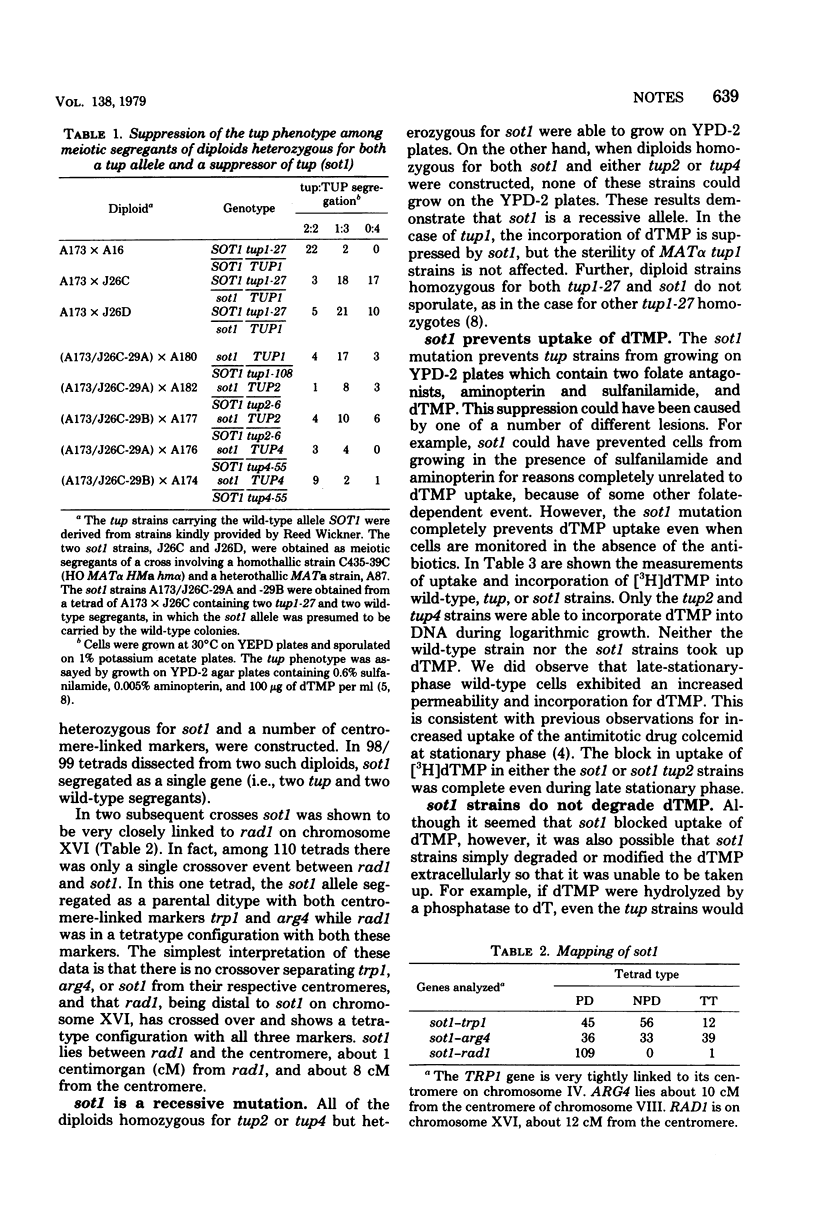
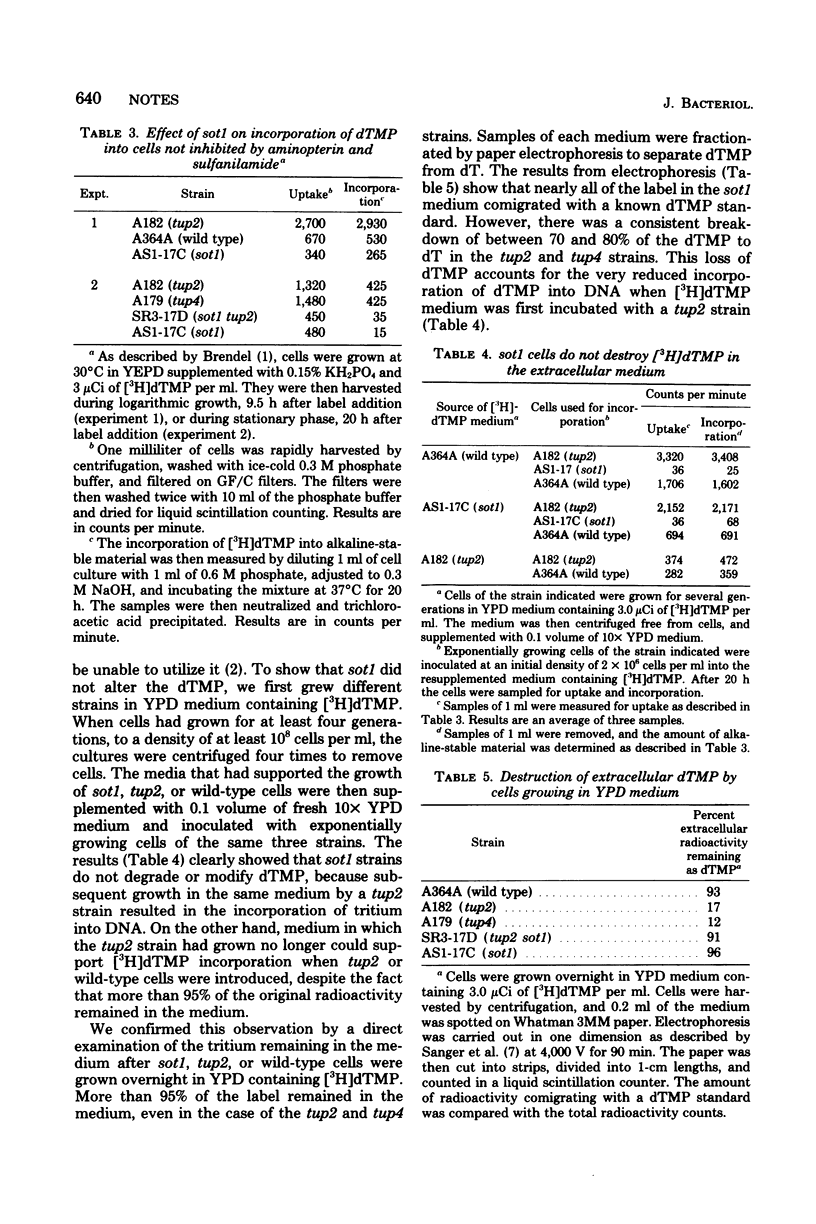
Although yeast cannot normally incorporate exogenous deoxythymidine 5'-monophosphate (dTMP) into deoxyribonucleic acid, mutants able to do so have been isolated. We have characterized a recessive suppressor of dTMP uptake (sot1) that prevents strains carrying either tup1, tup2, or tup4 from growing on selective medium. The sot1 mutation maps between rad1 and the centromere of chromosome XVI, and is unlinked to any of the tup mutations. The sot1 mutation does not suppress the other pleiotropic effects of the tup1 mutant, notably the lack of mating of tup1 MATalpha strains. The sot1 mutation specifically blocks the uptake of dTMP into tup strains. After growing a sot1 strain in medium containing [3H]dTMP, we showed that the medium still contained more than 90% of the original [3H]dTMP and that this medium could support the incorporation of [3H]dTMP by a tup2 strain. Therefore, sot1 strains do not degrade dTMP in the medium. The sot1 mutation had no effect on the uptake of other nutrients essential for growth, including several amino acids, adenine, and uracil.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brendel M. A simple method for the isolation and characterization of thymidylate uptaking mutants in Saccharomyces cerevisiae. Mol Gen Genet. 1976 Aug 19;147(2):209–215. doi: 10.1007/BF00267573. [DOI] [PubMed] [Google Scholar]
- Brendel M., Haynes R. H. Exogenous thymidine 5'-monophosphate as a precursor for DNA synthesis in yeast. Mol Gen Genet. 1973 Nov 22;126(4):337–348. doi: 10.1007/BF00269443. [DOI] [PubMed] [Google Scholar]
- Grivell A. R., Jackson J. F. Thymidine kinase: evidence for its absence from Neurospora crassa and some other micro-organisms, and the relevance of this to the specific labelling of deoxyribonucleic acid. J Gen Microbiol. 1968 Dec;54(2):307–317. doi: 10.1099/00221287-54-2-307. [DOI] [PubMed] [Google Scholar]
- Haber J. E., Peloquin J. G., Halvorson H. O., Borisy G. G. Colcemid inhibition of cell growth and the characterization of a colcemid-binding activity in Saccharomyces cerevisiae. J Cell Biol. 1972 Nov;55(2):355–367. doi: 10.1083/jcb.55.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannsen S., Witte I., Megnet R. Mutants for the specific labelling of DNA in Saccharomyces cerevisiae. Biochim Biophys Acta. 1973 Apr 11;299(4):681–685. doi: 10.1016/0005-2787(73)90243-8. [DOI] [PubMed] [Google Scholar]
- Lemontt J. F. Pathways of ultraviolet mutability in Saccharomyces cerevisiae. III. Genetic analysis and properties of mutants resitant to ultraviolet-induced forward mutation. Mutat Res. 1977 May;43(2):179–204. doi: 10.1016/0027-5107(77)90003-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Wickner R. B. Mutants of Saccharomyces cerevisiae that incorporate deoxythymidine-5'-monophosphate into deoxyribonucleic acid in vivo. J Bacteriol. 1974 Jan;117(1):252–260. doi: 10.1128/jb.117.1.252-260.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



