Abstract
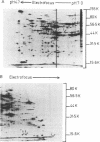
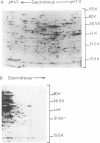
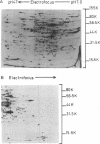
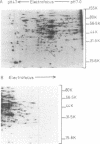
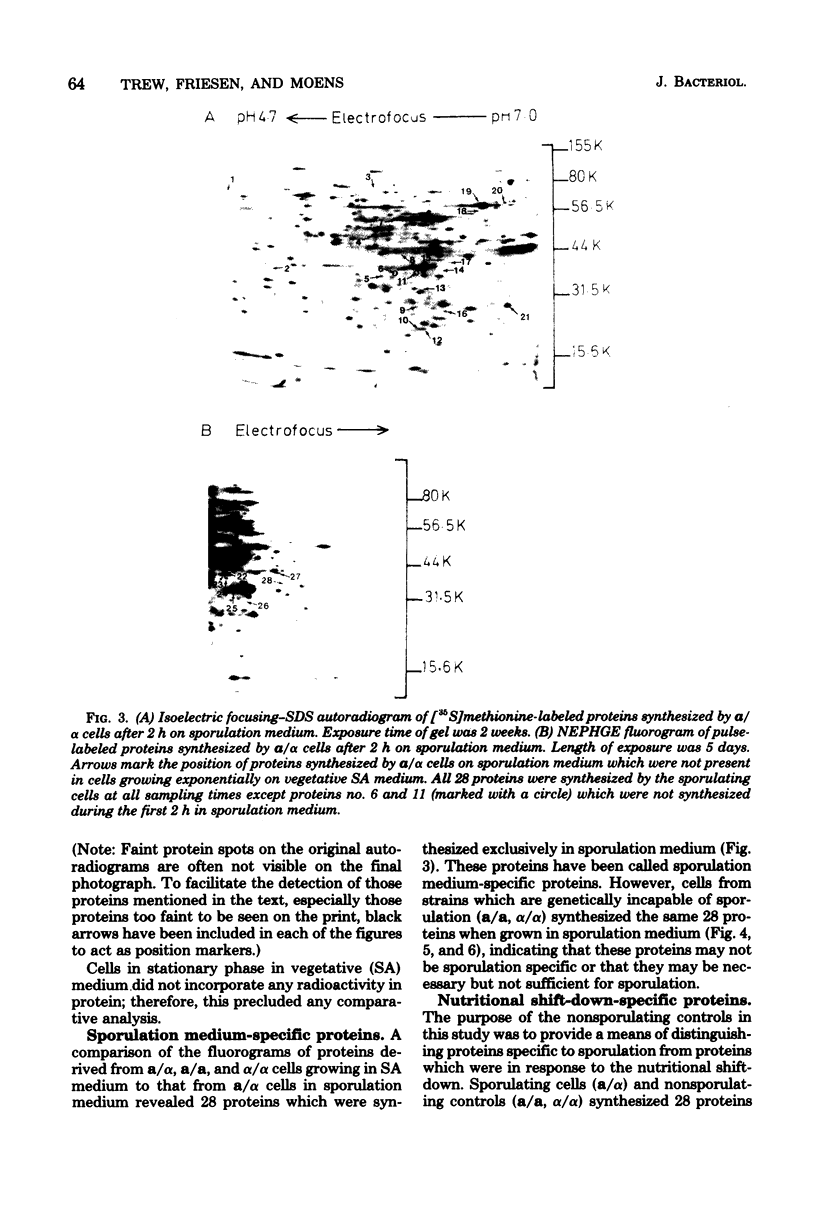
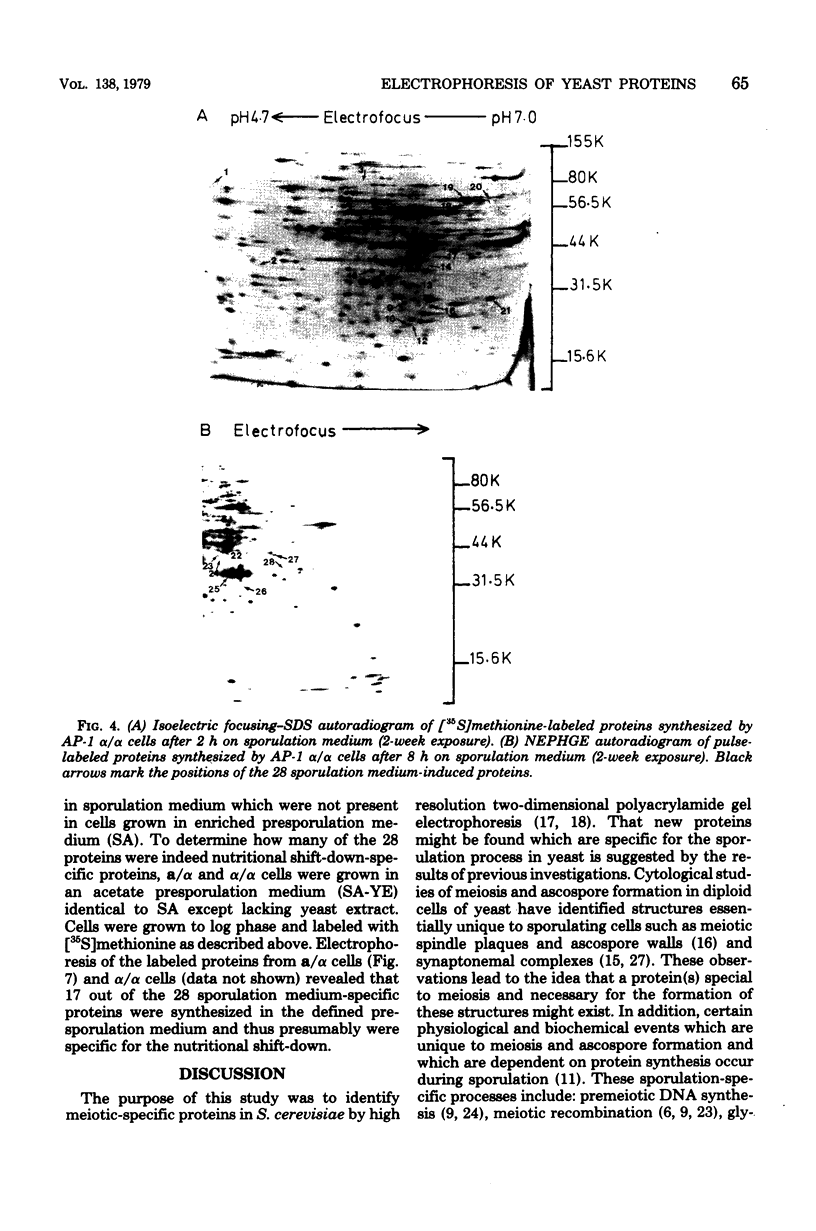
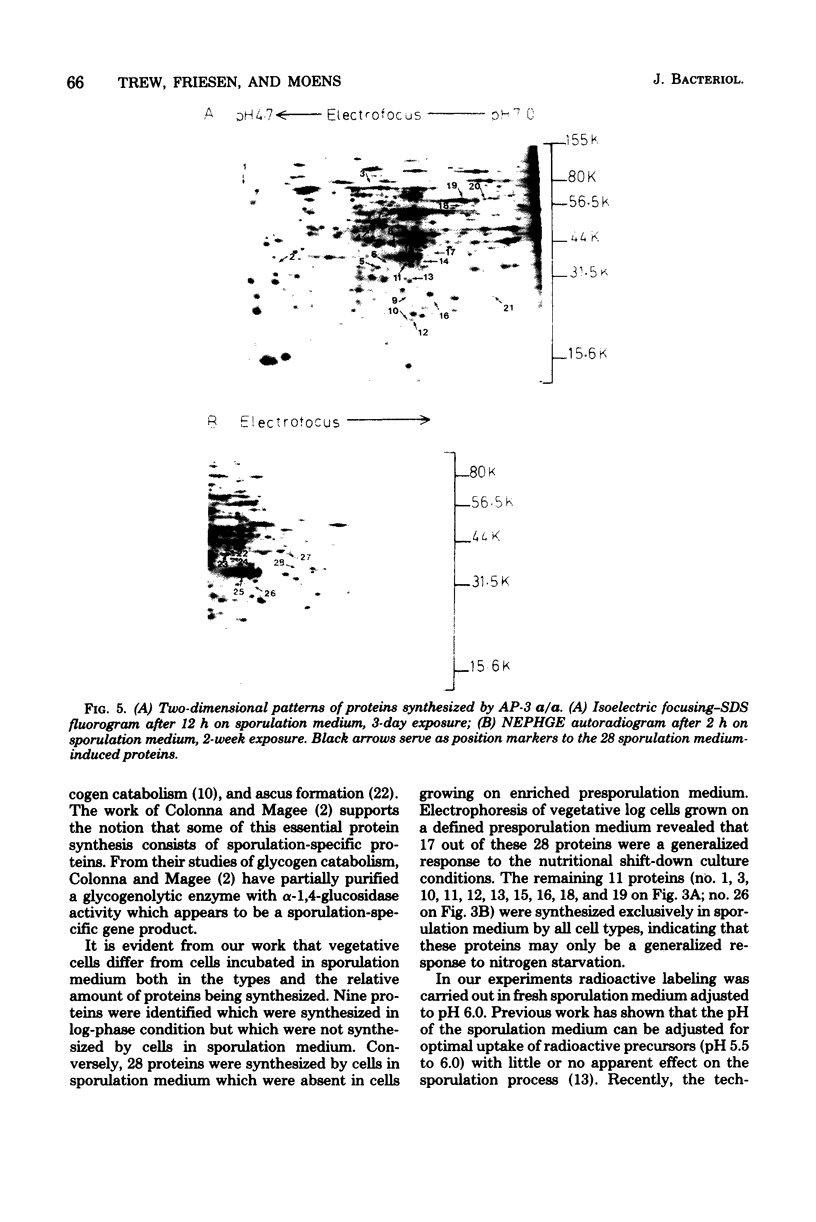
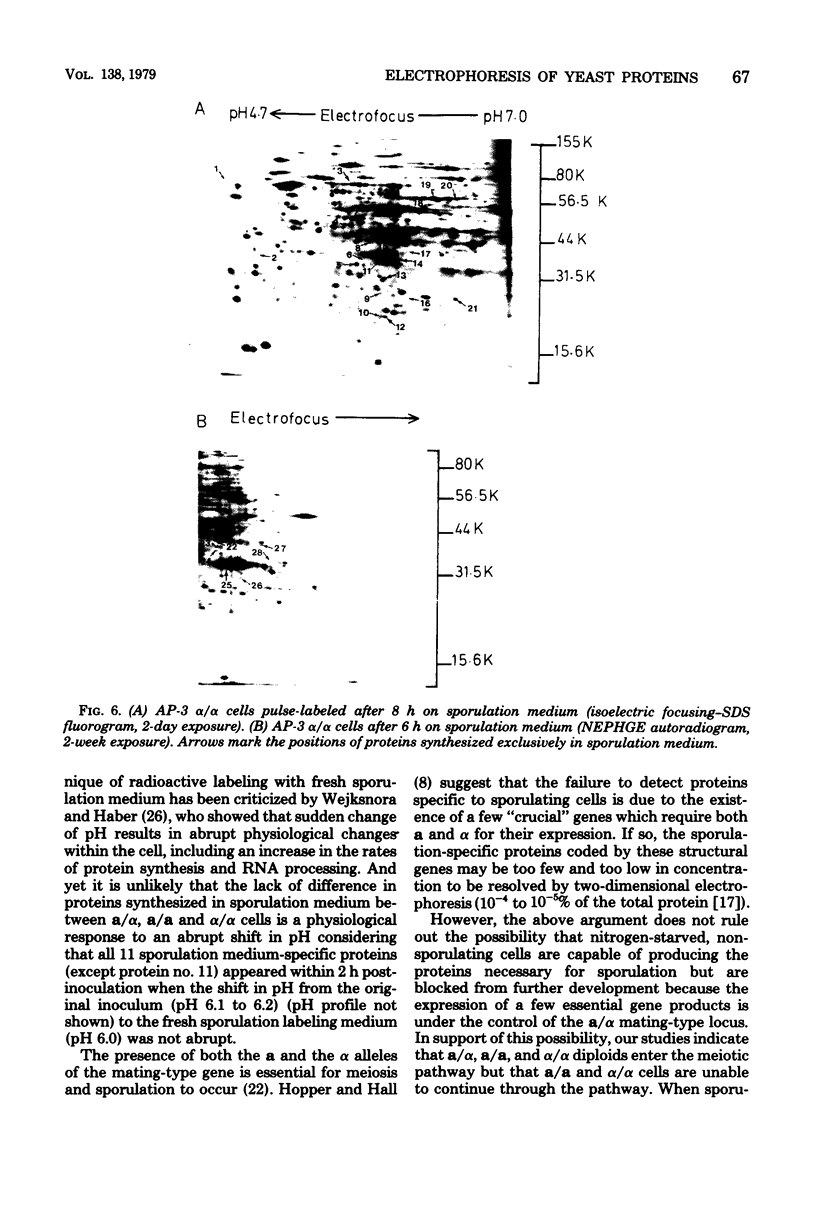
Proteins synthesized by Saccharomyces cerevisiae in presporulation and sporulation media were compared by using sporulating (a/alpha) and nonsporulating (a/a and alpha/alpha) yeast strains. Total cellular proteins were labeled with [35S]methionine and analyzed by two-dimensional polyacrylamide gel electrophoresis. Autoradiograms and/or fluorograms showed some 700 spots per gel. Nine proteins were synthesized by a/alpha cells which were specific to vegetative, log-phase conditions. During incubation in sporulation medium, sporulating (a/alpha) cells synthesized 11 proteins not present in vegetatively growing cell. These same 11 proteins, however, were synthesized by nonsporulating (a/a and alpha/alpha) cells on sporulation medium as well. Nonsporulating diploids (a/a and alpha/alpha) were also examined with the electron microscope at various times during their incubation in sporulation medium. Certain cellular responses found to be unique to meiotic yeast cells in previous studies were exhibited by the nonsporulating controls. The degree to which all cell types (a/alpha, a/a, and alpha/alpha) were committed to sporulation was also determined by shifting cells from sporulation medium to vegetative medium. Some commitment to the meiotic pathway was observed in both the a/alpha and the a/a, alpha/alpha cells.
Full text
PDF

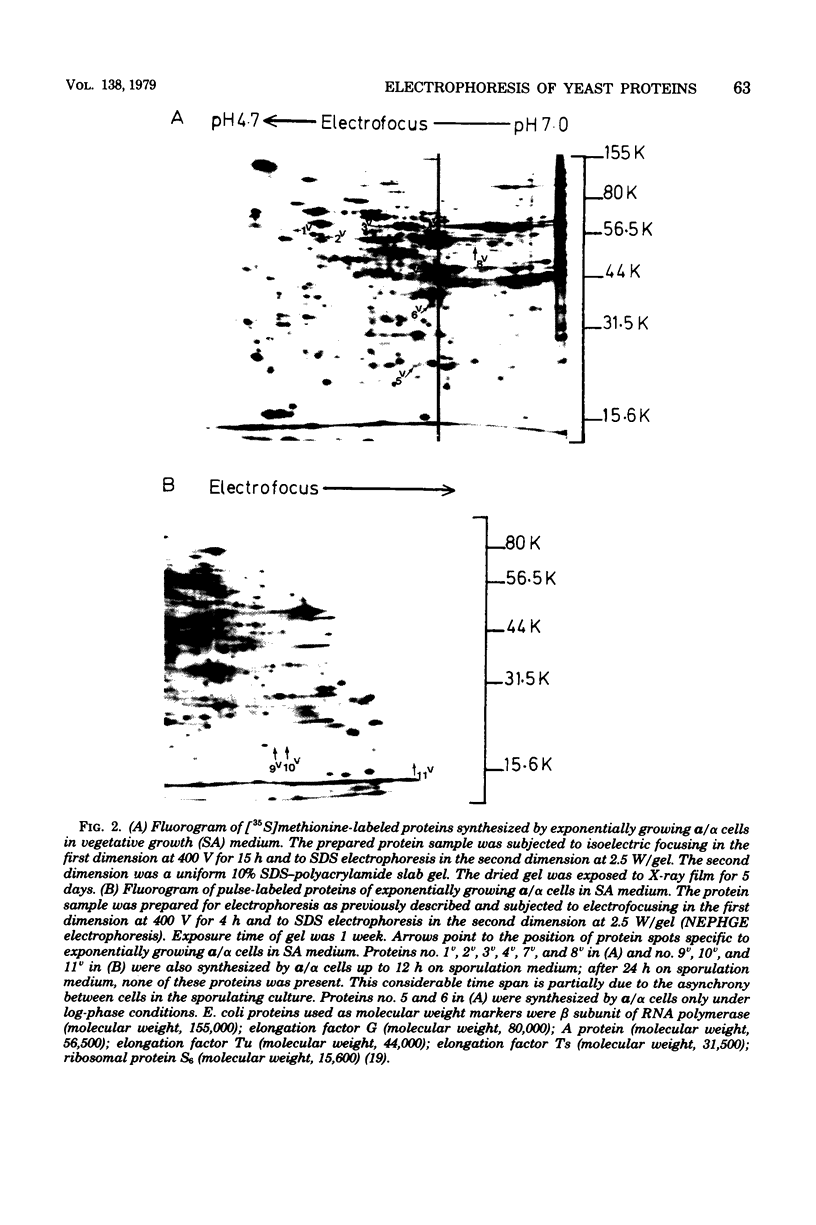

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Colonna W. J., Magee P. T. Glycogenolytic enzymes in sporulating yeast. J Bacteriol. 1978 Jun;134(3):844–853. doi: 10.1128/jb.134.3.844-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito M. S., Esposito R. E. The genetic control of sporulation in Saccharomyces. I. The isolation of temperature-sensitive sporulation-deficient mutants. Genetics. 1969 Jan;61(1):79–89. doi: 10.1093/genetics/61.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fast D. Sporulation synchrony of Saccharomyces cerevisiae grown in various carbon sources. J Bacteriol. 1973 Nov;116(2):925–930. doi: 10.1128/jb.116.2.925-930.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis J., Roman H. The effect of the mating-type alleles on intragenic recombination in yeast. Genetics. 1968 May;59(1):33–36. doi: 10.1093/genetics/59.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H. Saccharomyces cerevisiae cell cycle. Bacteriol Rev. 1974 Jun;38(2):164–198. doi: 10.1128/br.38.2.164-198.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper A. K., Hall B. D. Mating type and sporulation in yeast. I. Mutations which alter mating-type control over sporulation. Genetics. 1975 May;80(1):41–59. doi: 10.1093/genetics/80.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper A. K., Magee P. T., Welch S. K., Friedman M., Hall B. D. Macromolecule synthesis and breakdown in relation to sporulation and meiosis in yeast. J Bacteriol. 1974 Aug;119(2):619–628. doi: 10.1128/jb.119.2.619-628.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane S. M., Roth R. Carbohydrate metabolism during ascospore development in yeast. J Bacteriol. 1974 Apr;118(1):8–14. doi: 10.1128/jb.118.1.8-14.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee P. T., Hopper A. K. Protein synthesis in relation to sporulation and meiosis in yeast. J Bacteriol. 1974 Sep;119(3):952–960. doi: 10.1128/jb.119.3.952-960.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker J. H., Haber J. E. Efficient sporulation of yeast in media buffered near pH6. J Bacteriol. 1977 Oct;132(1):180–185. doi: 10.1128/jb.132.1.180-185.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills D. Effect of pH on adenine and amino acid uptake during sporulation in Saccharomyces cerevisiae. J Bacteriol. 1972 Oct;112(1):519–526. doi: 10.1128/jb.112.1.519-526.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens P. B. Fine structure of ascospore development in the yeast Saccharomyces cerevisiae. Can J Microbiol. 1971 Apr;17(4):507–510. doi: 10.1139/m71-084. [DOI] [PubMed] [Google Scholar]
- Moens P. B., Rapport E. Spindles, spindle plaques, and meiosis in the yeast Saccharomyces cerevisiae (Hansen). J Cell Biol. 1971 Aug;50(2):344–361. doi: 10.1083/jcb.50.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens P. B., Rapport E. Synaptic structures in the nuclei of sporulating yeast, Saccharomyces cerevisiae (Hansen). J Cell Sci. 1971 Nov;9(3):665–677. doi: 10.1242/jcs.9.3.665. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Pedersen S., Bloch P. L., Reeh S., Neidhardt F. C. Patterns of protein synthesis in E. coli: a catalog of the amount of 140 individual proteins at different growth rates. Cell. 1978 May;14(1):179–190. doi: 10.1016/0092-8674(78)90312-4. [DOI] [PubMed] [Google Scholar]
- Pedersen S., Reeh S. V. Analysis of the proteins synthesized in ultraviolet light-irradiated Escherichia coli following infection with the bacteriophages lambdadrifd 18 and lambdadfus-3. Mol Gen Genet. 1976 Mar 30;144(3):339–343. doi: 10.1007/BF00341733. [DOI] [PubMed] [Google Scholar]
- Rapport E. Some fine structure features of meiosis in the yeast Saccharomyces cerevisiae. Can J Genet Cytol. 1971 Mar;13(1):55–62. doi: 10.1139/g71-008. [DOI] [PubMed] [Google Scholar]
- Roman H., Sands S. M. Heterogeneity of Clones of Saccharomyces Derived from Haploid Ascospores. Proc Natl Acad Sci U S A. 1953 Mar;39(3):171–179. doi: 10.1073/pnas.39.3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R., Fogel S. A system selective for yeast mutants deficient in meiotic recombination. Mol Gen Genet. 1971;112(4):295–305. doi: 10.1007/BF00334431. [DOI] [PubMed] [Google Scholar]
- Roth R., Lusnak K. DNA synthesis during yeast sporulation: genetic control of an early developmental event. Science. 1970 Apr 24;168(3930):493–494. doi: 10.1126/science.168.3930.493. [DOI] [PubMed] [Google Scholar]
- Simchen G., Piñon R., Salts Y. Sporulation in Saccharomyces cerevisiae: premeiotic DNA synthesis, readiness and commitment. Exp Cell Res. 1972 Nov;75(1):207–218. doi: 10.1016/0014-4827(72)90538-1. [DOI] [PubMed] [Google Scholar]
- Wejksnora P., Haber J. E. Influence of pH on the rate of ribosomal ribonucleic acid synthesis during sporulation in Saccharomyces cerevisiae. J Bacteriol. 1976 Jul;127(1):128–134. doi: 10.1128/jb.127.1.128-134.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zickler D., Olson L. W. The synaptonemal complex and the spindle plaque during meiosis in yeast. Chromosoma. 1975;50(1):1–23. doi: 10.1007/BF00284959. [DOI] [PubMed] [Google Scholar]