Abstract
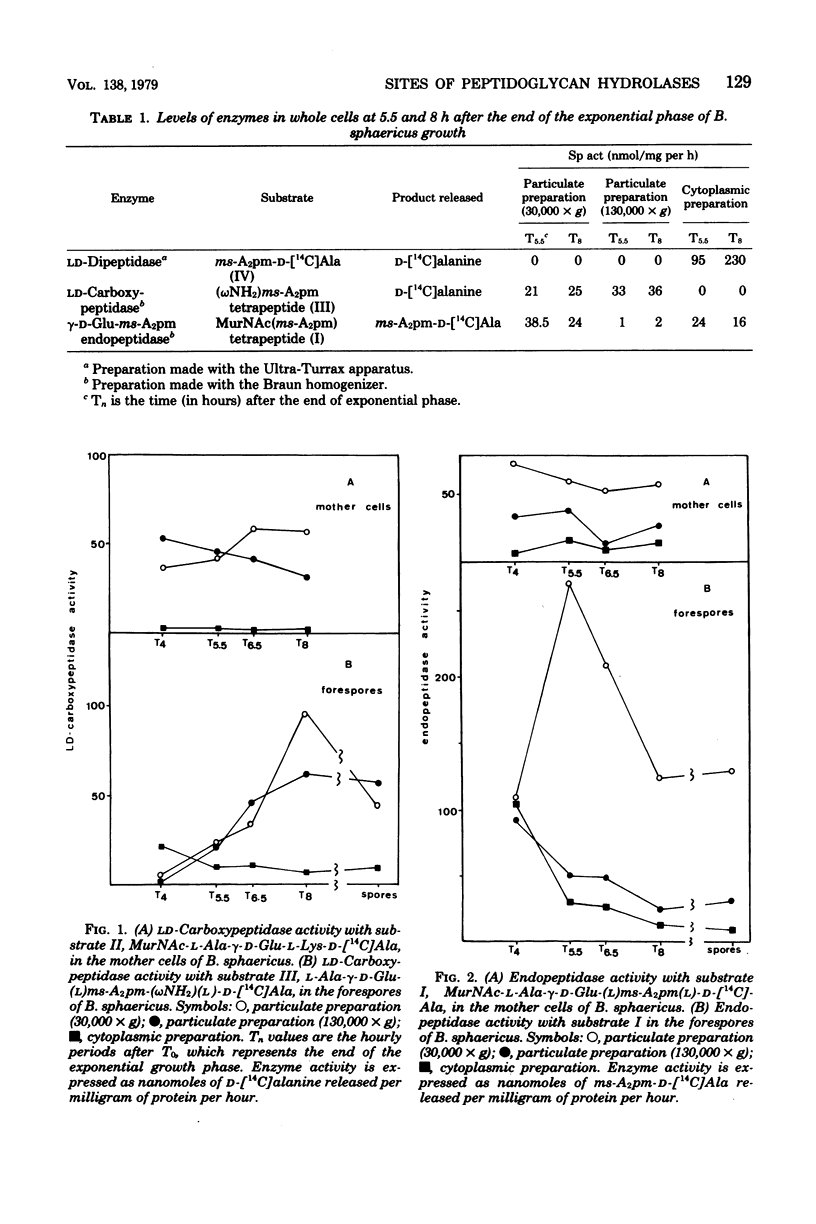
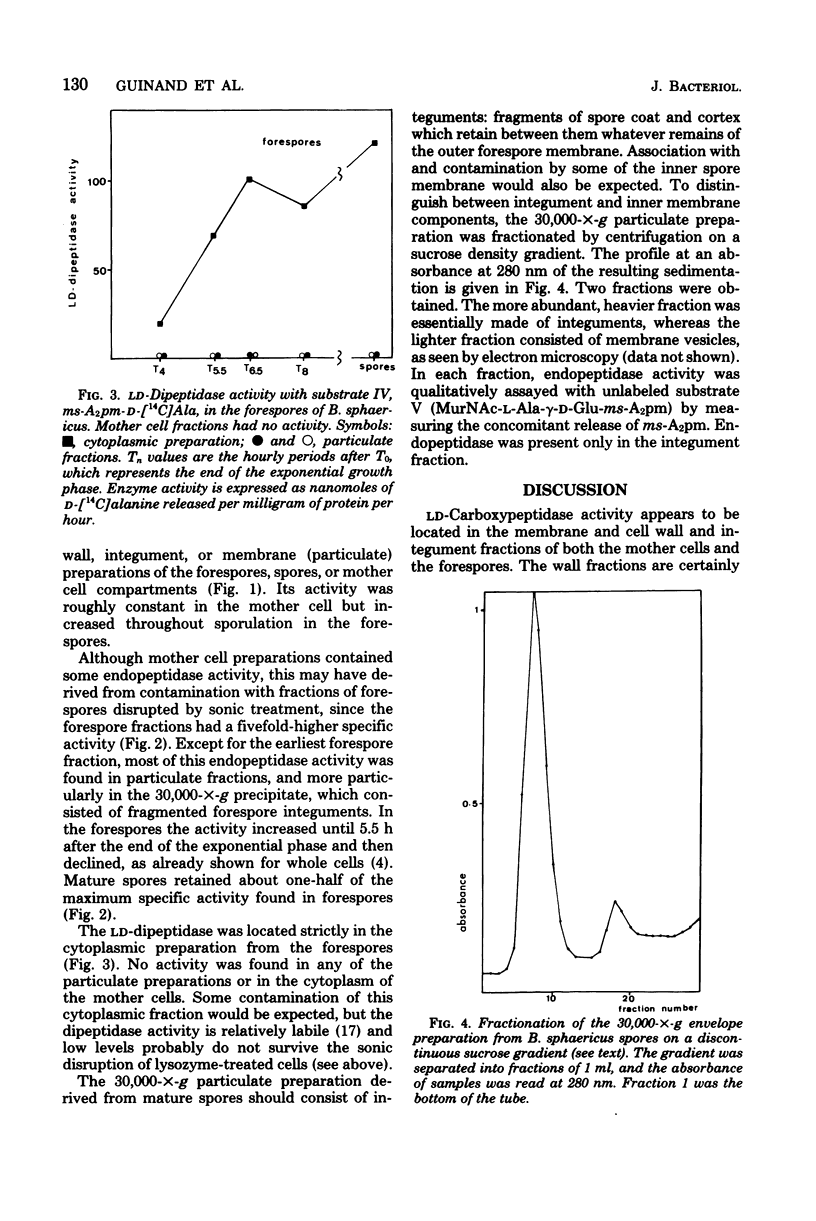
The level of three peptidoglycan hydrolases was determined in the mother cell compartment and forespores of Bacillus sphaericus. Vegetative and sporulating cells contained in LD-carboxypeptidase active only on the vegetative cell wall peptidoglycan, and we have previously shown that sporulation is accompanied by the production of two new enzymes active only on the spore cortex peptidoglycan. These gamma-D-glutamyl-meso-diaminopimelate endopeptidase and a meso-diaminopimelate-D-alanine dipeptidase. The LD-carboxypeptidase activity appeared to be located in the membranes of both the mother cells and forespores. Endopeptidase activity was located in the integument fraction of the forespores, and the dipeptidase activity was only found in the forespore cytoplasm. These different locations comply with the probable different functions of these enzymes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arminjon F., Guinand M., Michel G., Coyette J., Ghuysen J. M. Préparation enzymatique de peptides du type L-Ala-D-Glu (L)meso-diaminopimélyl(L)-D-Ala(14C) par réactions d'échange entre les peptides correspondants non radioactifs et la D-alanine (14C) Biochimie. 1976;58(10):1167–1172. doi: 10.1016/s0300-9084(76)80115-0. [DOI] [PubMed] [Google Scholar]
- Arminjon F., Guinand M., Vacheron M. J., Michel G. Specificity profiles of the membrane-bound gamma-D-glutamyl-(L)meso-diaminopimelateendopeptidase and LD-carboxypeptidase from Bacillus sphaericus 9602. Eur J Biochem. 1977 Mar 1;73(2):557–565. doi: 10.1111/j.1432-1033.1977.tb11351.x. [DOI] [PubMed] [Google Scholar]
- Guinand M., Michel G., Balassa G. Lytic enzymes in sporulating Bacillus subtilis. Biochem Biophys Res Commun. 1976 Feb 23;68(4):1287–1293. doi: 10.1016/0006-291x(76)90336-3. [DOI] [PubMed] [Google Scholar]
- Guinand M., Michel G., Tipper D. J. Appearance of gamma-D-glutamyl-(L) meso-diaminopimealate peptidoglycan hydrolase during sporulation in Bacillus sphaericus. J Bacteriol. 1974 Oct;120(1):173–184. doi: 10.1128/jb.120.1.173-184.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinand M., Vacheron M. J., Michel G. Relation between inhibition of Bacilli sporulation and synthesis of lytic enzymes. Biochem Biophys Res Commun. 1978 Jan 30;80(2):429–434. doi: 10.1016/0006-291x(78)90695-2. [DOI] [PubMed] [Google Scholar]
- Holt S. C., Gauther J. J., Tipper D. J. Ultrastructural studies of sporulation in Bacillus sphaericus. J Bacteriol. 1975 Jun;122(3):1322–1338. doi: 10.1128/jb.122.3.1322-1338.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungerer K. D., Tipper D. J. Cell wall polymers of Bacillus sphaericus 9602. I. Structure of the vegetative cell wall peptidoglycan. Biochemistry. 1969 Sep;8(9):3577–3587. doi: 10.1021/bi00837a013. [DOI] [PubMed] [Google Scholar]
- Kingan S. L., Ensign J. C. Isolation and characterization of three autolytic enzymes associated with sporulation of Bacillus thuringiensis var. thuringiensis. J Bacteriol. 1968 Sep;96(3):629–638. doi: 10.1128/jb.96.3.629-638.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Schnaitman C. A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970 Nov;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. P., Setlow B., Setlow P. Levels of small molecules and enzymes in the mother cell compartment and the forespore of sporulating Bacillus megaterium. J Bacteriol. 1977 Jun;130(3):1130–1138. doi: 10.1128/jb.130.3.1130-1138.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper D. J., Linnett P. E. Distribution of peptidoglycan synthetase activities between sporangia and forespores in sporulating cells of Bacillus sphaericus. J Bacteriol. 1976 Apr;126(1):213–221. doi: 10.1128/jb.126.1.213-221.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper D. J., Pratt I. Cell wall polymers of Bacillus sphaericus 9602. II. Synthesis of the first enzyme unique to cortex synthesis during sporulation. J Bacteriol. 1970 Aug;103(2):305–317. doi: 10.1128/jb.103.2.305-317.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacheron M. J., Guinand M., Arminjon F., Michel G. Mise en évidence et séparation d'une gamma-D-glutamyl-(L)meso-diaminopimélate endopeptidase et d'une LD-carboxypeptidase exocellulaires à partir de Bacillus sphaericus 9602. Biochimie. 1977;59(1):15–21. doi: 10.1016/s0300-9084(77)80081-3. [DOI] [PubMed] [Google Scholar]
- Wartell R. M., Larson J. E., Wells R. D. Netropsin. A specific probe for A-T regions of duplex deoxyribonucleic acid. J Biol Chem. 1974 Nov 10;249(21):6719–6731. [PubMed] [Google Scholar]
- Warth A. D., Strominger J. L. Structure of the peptidoglycan from spores of Bacillus subtilis. Biochemistry. 1972 Apr 11;11(8):1389–1396. doi: 10.1021/bi00758a010. [DOI] [PubMed] [Google Scholar]
- Warth A. D., Strominger J. L. Structure of the peptidoglycan from vegetative cell walls of Bacillus subtilis. Biochemistry. 1971 Nov 23;10(24):4349–4358. doi: 10.1021/bi00800a001. [DOI] [PubMed] [Google Scholar]



