Abstract
Persistent contradictions in well supported empirical findings usually point to important scientific problems and may even lead to exciting new insights. One of the most enduring problems in evolutionary biology is the apparent conflict between paleontological and embryological evidence regarding the homology of the digits in the avian hand (1, 2). We propose that this problem highlights an important feature of morphological change: namely, the possible dissociation between the developmental origin of a particular repeated element and its subsequent individualization into a fully functional character. We argue that, although comparative embryological evidence correctly identifies the homology of the primordial condensations in avians as CII, CIII, and CIV, subsequent anatomical differentiation reflects a frame shift in the developmental identities of the avian digit anlagen in later ontogeny such that CII becomes DI, CIII becomes DII, and CIV becomes DIII.
An Enduring Problem
Assigning homology to the three fingers in the hand of living birds is a problem that has challenged morphologists since well before the Theory of Descent was first proposed (e.g., refs. 3 and 4; see reviews in refs. 2 and 5–7). The morphology of well preserved and fully formed hands of an early maniraptoran theropod dinosaur from the late Jurassic, Archaeopteryx lithographica (Avialae; Fig. 1), led many to accept the identity of the three fingers as being digits I, II, and III (Fig. 2b). Some embryologists, however, countered this hypothesis, instead producing empirical evidence in early hand morphogenesis suggesting that the three avian digits are actually II, III and IV. Strikingly, this debate has endured to the present day (1).
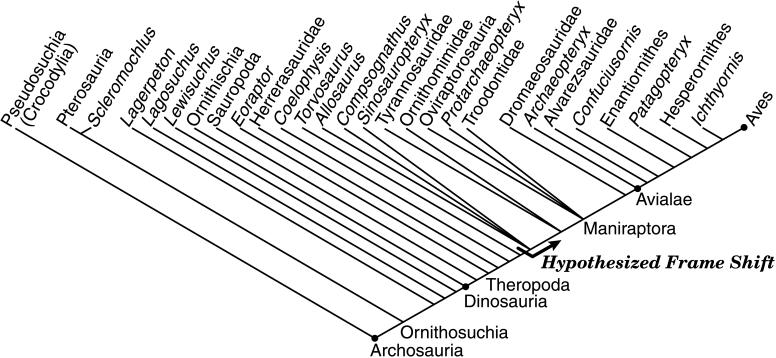
Figure 1.
Consensus cladogram depicting phylogenetic relationships among select bird-line archosaurs based on refs. 2 and 32–35, showing stem along which proposed frame shift is hypothesized to have occurred. Archosauria, node-based name for the crown clade stemming from the last common ancestor Aves shared with Crocodylia; Ornithosuchia, stem-based name for Aves plus all other archosaurs more closely related to Aves than to Crocodylia; Dinosauria, node-based name for the clade stemming from the last common ancestor shared by Aves and Ornithischia; Theropoda, stem-based name for the clade including Aves and all dinosaurs more closely related to Aves than to Sauropoda; Maniraptora, stem-based name for the clade including Aves and all theropods more closely related to Aves than to Ornithomimidae; Avialae, node-based name for the clade stemming from the last common ancestor shared by Archaeopteryx and Aves; Aves, node-based name for the crown clade stemming from the last common ancestor shared by Palaeognathae and Neognathae.
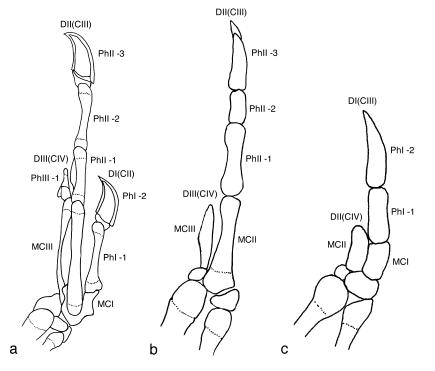
Figure 2.
Dorsal view of left hand of maniraptor theropods. (a) Deinonychus antirrhopus. Shown is a composite of YPM 5206 (manus) and YPM 5208 (semilunate carpal). (b) Archaeopteryx lithographica (Berlin specimen). (c) Nothura maculosa (YPM-OST 2086; DI is missing the ungual phalanx). DI—III, digits; C1–3, distal carpals comprising semilunate carpal; r, radiale.
In this paper, we first review paleontological and embryological evidence that support and contradict, respectively, the I, II, III hypothesis and then proceed to review data regarding the phylogenetic position of birds. Based on this review, we conclude that the conflict between paleontological and embryological data is real; it can be resolved neither by rejecting one kind of data nor by assuming that birds are not theropod dinosaurs. We argue that early theropod dinosaurs faced a conflict between the functional constraints favoring the retention of digits I, II, and III and the developmental constraints that favored the loss of the condensation that normally develops into the first finger. We propose that, after the secondary loss of digit DIV, a frame shift in digit identity occurred that forced condensation CII into the developmental pathway of digit DI, condensation CIII into digit DII, etc.
Anatomical and Functional Evidence in Support of the I, II, III-Hypothesis
Archaeopteryx (Fig. 2b) has a three-fingered hand with a phalangeal formula (2-3-4) diagnostic of the inner three digits (DI, DII, DIII). That phalangeal formula is already present in Tulerpeton (8), and it is thus a synapomorphy that arose in the late Devonian, before the origin of Tetrapoda. That ancestral phalangeal formula persists, at least in digits DI and DII (the thumb and index fingers), into basal Aves [e.g., the palaeognath Struthio (9) and the neognath Opisthocomus (10) (Figs. 2c and 5a)].
Figure 5.
Dorsal view of left hands of late embryos of Hoatzin (Opisthocomus: Neognathae) after ref. 7 (a) and Kiwis (Apteryx: Palaeognathae) after ref. 10 (b and c). DI–III, digits; CII–IV, condensations; MCI–III, metacarpals.
It has long been known that the hand in Archaeopteryx matches closely the hand form common to closely related theropod dinosaurs such as Deinonychus (Fig. 2; refs. 2, 11, and 12). Although capable of flight, Archaeopteryx still retains the raptorial hands characteristic of basal maniraptor theropods. That is to say, Archaeopteryx is like other theropods in having elongate penultimate phalanges on the inner three fingers that bear the unguals supporting large, deeply recurved, sharply pointed, prehensile claws (Fig. 2; ref. 2).
Quite apart from phalangeal formula, each bony element in the theropod hand is easily distinguished by the relative size and shape of its shaft and the morphology of its articular surfaces. For example, the penultimate phalanges are especially long, as mentioned above, particularly in the thumb and index fingers (Fig. 2). Note also that the second finger in the hand is the longest in birds (Fig. 2c), as that synapomorphy arose within Theropoda. The third finger in maniraptors such as Deinonychus and Archaeopteryx is equally noteworthy for bearing two short, interlocking phalanges proximally that articulate with a long penultimate phalanx. That phalanx is unique for twisting nearly 50° about its long axis to articulate with the claw-bearing ungual, which now points inwards (Fig. 2a).
Avian fingers are generally reduced and highly modified (Fig. 2c), but the hand still approaches (or exceeds) the size of the foot as it does in closely related theropods, such as Deinonychus (2). And even if we ignore finger morphology and phalangeal formula, the metacarpals comprising the palm of the avian hand still exhibit marks of their dinosaurian heritage (Fig. 3; refs. 2 and 12–14). A tiny fifth metacarpal is, for example, displaced to the palmar surface of the hand, and it is absent in postembryonic stages from Coelophysis through Aves. Even in the earliest theropods, the fourth and fifth metacarpals are vestigial and do not project from the palm of the hand, so that, although they are technically pentadactyl, they are functionally tridactyl, just like modern-day birds. The trend to reduction of the posterior hand margin continues within theropods, as the fourth metacarpal is lost and the third metacarpal becomes shorter than the second. And maniraptor theropods such as Deinonychus are especially bird-like in that the second and third metacarpals are long and gracile and the third metacarpal is thin, bowed, and displaced ventrally [the flight feathers pass above the third metacarpal to attach to the second in Archaeopteryx and Aves (15)].
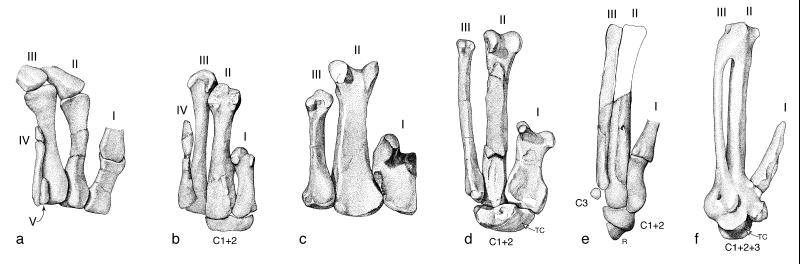
Figure 3.
Ventral view of metacarpals and select wrist bones in palm of theropod hand. (a) Herrerasaurus ischigualastensis (after ref. 58); left metacarpals (reversed). (b) Coelophysis bauri (MCZ 4331); right metacarpals and compound distal carpal. (c) Allosaurus fragilis (YPM 4944); left metacarpals (reversed). (d) Deinonychus antirrhopus (YPM 5206); left metacarpals and YPM 5208, left semilunate carpal (reversed). (e) Archaeopteryx lithographica (Eichstätt specimen); left metacarpals and wrist bones. (f) Nothura maculosa (YPM-OST 2086); left carpometacarpus (reversed). I–V, metacarpals; C1–3, distal carpals; r, radiale; TC, trochlea carpalis.
The first metacarpal has offset heads that direct the thumb (DI) away from the other fingers in dinosaurs ancestrally (Fig. 3). The thumb (DI) and middle finger (DIII) have important functional implications because these fingers spread apart when the hand is opened and converge when it is closed, thus enhancing the predatory role of the hands in the two-armed grasp presumed ancestral for maniraptors (2, 14, 16, 17). Part of this original functional complex is retained in Aves, as an independently mobile thumb is essential to the function of the alula that it supports (2, 18).
The first and second distal carpals are fused into a compound element in theropods other than herrerasaurs, and the third distal carpal is later incorporated into this structure in avialan phylogeny (and in avian ontogeny). In maniraptors, this compound carpal bone is half-moon-shaped, and it bears a uniquely avian articular surface proximally: the trochlea carpalis (Fig. 3 d and f). This “semilunate” carpal caps metacarpals I and II, unifying them functionally within the wrist.
Embryological Data in Conflict with the I, II, III-Hypothesis
There has long been a dissenting view from the hypothesis that the bird hand is composed of digits DI, DII, and DIII. This position is held chiefly by embryologists who argue that the remaining fingers actually represent DII, DIII, and DIV because the DI and DV were thought to have been lost. Morse (19) observed that, when digital reduction occurs in mammals and lizards, the first digit (DI) is invariably the first to be lost in ontogeny, followed by the fifth (DV), and that a modified version of this pattern applies to the foot of birds as well. Thus, the proposition that ultimately became known as Morse’s Law holds that the three functional fingers remaining in adult birds must be DII, DIII, and DIV.
A century later, Hinchliffe (20, 21) relied on topographic relations among chondrogenic condensations in the postaxial limb bud to reach the same conclusion. We agree that Hinchliffe correctly identified the posterior hand margin because of the position of the fourth and fifth condensations. Hinchliffe’s (21) illustrations demonstrate that the fifth condensation, followed by the fourth, moves onto the palmar surface of the hand during avian ontogeny. The vestiges of the fourth and fifth digits in basal theropods such as Herrerasaurus (Fig. 3a) also lie on the palmar surface of the hand. Thus, because there are only four condensations in the avian hand, the two condensations in front of the posterior, and ventralmost, two condensations in the hand, which we agree are CV and CIV, must be CIII and CII.
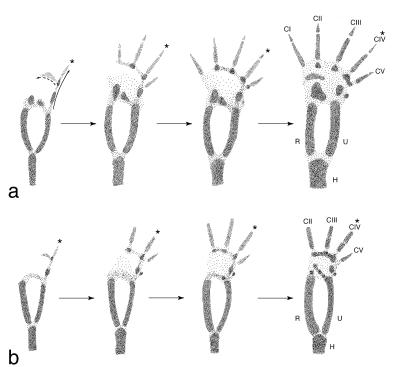
The landmark work of Pere Alberch and collaborators (22–26) provides a separate line of evidence supporting this view. They observed a conserved pattern of connections among primordial condensations within an embryonic limb bud to identify a primary limb axis (i.e., metapterygial axis; Fig. 4) (26). In five-fingered tetrapods, that axis passes down a chain of condensations ending in the fourth finger (Fig. 4a). Because there are only two condensations medial to that axis in embryonic birds, they are identified as the precursors of digits DII and DIII (Fig. 4b). Digit DI never appears. If any digits are to be lost, the first is a likely candidate because the condensation (CI) associated with the first finger is the last to form in the digital arch (24, 25). This phenomenon has been confirmed experimentally by injecting mitosis inhibitors into alligator eggs (27).
Figure 4.
Semidiagrammatic representations of developmental sequences from forelimbs of Alligator after ref. 23 (a) and Ostrich after ref. 22 (b) showing patterns of connectivity among condrogenic condensations. CI–CV, condensations; ∗, metapterygial axis.
Phylogenetic Relationships of Birds
One solution to the problem of avian digit homology in favor of the II, III, IV hypothesis is to assume that birds are not theropod dinosaurs (1, 5, 21). Removing birds from theropods implies that the similarities between the hands of Archaeopteryx and that of theropods are convergent and not homologous. This is, however, not a very satisfactory explanation for the available data. The hypothesis that birds are but one clade of theropod dinosaurs has survived repeated, extensive, and vigorous challenge since it first was proposed 136 years ago (28), and it has garnered additional support with each new fossil find (29–31).
The general outlines of theropod phylogeny during the Mesozoic are reasonably well understood, although the hypothesized relationships of several taxa remain contentious (Fig. 1; refs. 2 and 32–35). Nevertheless, alternative hypotheses of digital homologies have no impact on the position of birds. In fact, Gauthier’s (2) basic tree topology is recovered whether or not hand characters are deleted from or added to the analysis (results not shown), even with uncritical acceptance of every objection to the character data raised by modern critics of the theropod hypothesis (e.g., ref. 36).
Dissociation Between Morphogenesis and Character Identity
Provided that both alternative hypotheses of homology are strongly supported by their respective data types, resolution of the conflict calls for a reexamination of the mechanistic assumptions underlying the interpretation of the conflicting data. Embryological and anatomical data regarding the homology of adult structures can only be in conflict if one assumes an invariant causal link between the embryological events supporting one hypothesis and the adult anatomical features supporting the other. But, in many instances, unambiguously homologous characters are known to develop via different developmental pathways (37). This calls into question either the homology concept itself (38, 39) or the assumption that the causal links among different levels in the developmental hierarchy are invariant in evolution (40, 41).
A direct link between morphogenesis and character identity may exist in some cases: Vertebrate tooth and mollusk shell development are good examples. But that may not be the case in other characters that undergo considerable cell turnover and/or extensive growth after morphogenesis (42). Such characters have been found to be stable even in the face of extensive variation in early developmental events, demonstrating that character identity can exhibit partial causal independence from early morphogenetic events (42). Building on Tabin’s (43) insight, we suggest causal independence between the morphogenetic processes that create successive condensations in the limb bud and the ensuing developmental individualization of those repeated elements as they become the functional fingers in the mature hand, thus permitting an opportunity for some degree of independent evolutionary change.
Note that many of the anatomical characters indicating that bird digits are DI, DII, and DIII develop later than the embryological characters that identify bird digit condensations as CII, CIII, and CIV. The embryological characters derive from sequential and topological relations among chondrogenic condensations that develop along the primary limb axis and then back to front in the digital arch ancestral to tetrapods (Fig. 4; ref. 22). In contrast, the anatomical characters used to determine morphological digit identity arise after the condensations for the distal carpals and metacarpals have been laid down and long after the transitory connections among the condensations have been broken.
Two types of processes need to be distinguished. There are well documented cases in which early morphogenesis and subsequent development of character identity are known to be mechanistically independent. The best documented example is the development of body segments in Drosophila. Mutant phenotypes identified by Nüsslein-Volhard and Wieshaus (44) demonstrate that there are two sets of genes: segmentation genes and homeotic genes. Segmentation genes are necessary to generate body segments with no implications as to the final identity assumed by those segments. Homeotic genes act after the segmentation genes and convey segment identity. Independence between early morphogenesis and the development of character identity are the prerequisites to homeotic transformation. Reported cases of homeosis invariably involve characters developing from repeated elements, such as arthropod appendages, body segments, somites, and flower parts, to name but a few.
If the origin of elements in early morphogenesis and their subsequent individualization can be causally independent, then the identity of a given structure—a somite, a segment, or a digit—may be subject to independent evolutionary change. This has been beautifully demonstrated by Burke et al. (45), who have shown that morphologically corresponding body regions (e.g., neck and thorax) in birds and mammals express the same combination of Hox genes regardless of the particular somites from which they develop. We suggest that just such a frame shift may be involved in the evolution of the avian hand.
Development and Variation of Digit Identity.
Little is known about the genetic mechanisms that confer digit identity (D) to condensations (C), but two types of evidence are relevant to the question of digit identity in birds. There is some circumstantial evidence for Hox gene involvement in the development of digit identity, and there are telling data from experimental and natural variation in digit identity.
Genes Influencing Digit Development.
Considerable progress has been made in recent years in uncovering the genetic cascades involved in the initiation of limb development, the outgrowth of the limb bud, and the determination of the main axes of the limb (46, 47). In contrast, relatively little is known of the relationship between gene activity and the morphology of the adult limb skeleton, particularly the digits (48–50). The problem seems to be that the best studied class of genes, the so-called Hox genes, are involved in both the initial morphogenesis (e.g., they influence condensation of digit anlagen) as well as the subsequent growth and differentiation of digit anlagen (51–53). Experimental manipulation of Hox gene activity thus often affects the number as well as the development of skeletal elements in the limb (49). So there do not appear to be “digit identity” genes as such; rather, digit identity is determined by a complex interaction among many genes (48). In some situations, however, changes in digit identity can be induced without changes in digit number; for instance, overexpression of HoxD-11 in the bird hindlimb induces a DII morphology in the most anterior digit (which develops from condensation CI) (54). This shows that, in principle, digit identity can be changed by altered gene activity and that the genetic “digit identity code” is likely to be complex.
Variation in Digit Identity.
Assuming a partial independence of early morphogenesis and the development of digit identity implies that variation in the number of condensations may have consequences for the morphological identity of the remaining digits. In amniotes and frogs, mitosis inhibition during early limb-bud development invariably leads to the loss of condensation CI, which is the last condensation to form in the digital arch. This has been shown experimentally in frogs (24, 25) and alligators (27). Remarkably, the innermost finger left in experimental Alligator embryos exhibits a mixture of features normal to both the first and second digits (27). The phalangeal formula is that of the second finger (i.e., three phalanges), but its metacarpal is shorter than the following one, and the proximal articulation of the metacarpal is identical to that of the first finger in the wild type. In the wild type, metacarpal I articulates with the centrale instead of a distal carpal, as the rest of the digits do, whereas, in the experimental hands, the former “DII” now articulates with the centrale (G. Müller, personal communication). Furthermore, in the wild type, metacarpal I is shorter than metacarpals II and III whereas metacarpal II is subequal to III in length. In experimental animals, digit DII thus experiences a partial transformation of its character identity simply because of the loss of the anteriormost condensation.
A similar shift in digit identity with condensation loss is evident in the hand of the vestigial wing in the Kiwi [Apteryx (55)]. Kiwis have only two fingers, and, in the absence (or near absence) of digit DI (CII), digit DII (CIII) sometimes can display the number and shape of phalanges natural to digit DI instead of digit DII or a combination of attributes of both fingers (Fig. 5 b and c). It is equally revealing that, in Kiwis, the third condensation (CIII) never gives rise to a third digit (DIII). Natural variation of this sort demonstrates unambiguously that there is no one-to-one relationship between digit identity (D) and condensation identity (C). That is to say, depending on the theropod in question, morphological features characteristic of a thumb (DI) can develop from any of three different condensations, that is, either CI (e.g., Herrerasaurus), CII (e.g., Archaeopteryx), or CIII (e.g., some Apteryx).
An Evolutionary Scenario
We suggest that the theropod lineage leading toward birds faced a conflict between two evolutionary constraints: a functional necessity to retain the inner three fingers—especially the thumb—and an opposing developmental constraint favoring loss of the last-formed and most anterior condensation. The trend toward increasing functional demands on digits DI, DII, and DIII, and reduction of DIV and DV, is foreshadowed in both the hands and feet of the earliest archosaurs. Asymmetry in the hand is especially marked in basal dinosaurs in which the ancestral phalangeal formula (2-3-4-5-3) has been reduced to 2-3-4-3-2 and is reduced even further in early theropods to 2-3-4-1/0-X and later to 2-3-4-X-X (2).
We thus envision an initial phase in which digital reduction in the theropod hand proceeded by secondary reduction; namely, the condensations are retained, but the growth and differentiation of the anlage of the fourth and fifth digits are retarded (Fig. 6 A and B). Once the fifth finger, and later the fourth, became reduced, the digital-development pathways used only four and then three of the original five condensations. At least one of the condensations became redundant during theropod evolution. Because condensation patterns are so conservative, it appears difficult to drop the fourth and fifth condensations from the developmental program without losing the first condensation (22). In fact, there is still a remnant of the fifth condensation that appears briefly in early bird-hand development; only the first condensation is completely absent (Fig. 4b; ref. 5). Hence, the only way to get rid of the redundant condensation is to delete the last one to form (CI). Once this happened, we postulate a frame shift—a homeotic transformation—in the developmental identity of the initial condensations: Condensation CII developed into digit DI, CIII developed into DII, and CIV developed into DIII (Fig. 6C), thus conserving a functionally significant mature form within the confines of a morphogenetic constraint.
Figure 6.
Hypothesis about the phylogenetic transformation of digit development leading from an ancestral five-digit theropod hand (e.g., Herrerasaurus) to the three-digit hand of Allosaurus and the maniraptorans including living birds. (A) Herrerasaurus has five digits (DI–DV), but only three are functional (DI–DIII, bold) and the remaining are vestigial (DIV and DV, outlined) (see also Fig. 3a). We assume that Herrerasaurus followed the usual amniote pattern in which condensation CI develops into digit DI, etc. (B) The next stage of evolution is the loss of digit DV, as exemplified by Coelophysis (see Figs. 1 and 3b). Coelophysis has three functional digits, DI–DIII, and one vestigial digit, DIV. We assume that this step is because of a secondary loss of digit DV in which condensation CV forms but fails to differentiate into a digit. Condensation CV still forms during hand development of living birds (1) but fails to fully chondrify and is eventually absorbed. Occasionally, a remnant of DV is found in a few individuals of Coelophysis (59), demonstrating that CV is retained from early theropods into living birds. (C) The transition from a four- to a three-digit hand, represented by Allosaurus, eventually leads to the loss of condensation CI, as shown in the development of living birds. During this transition, we hypothesize a frame shift that forces CII in to the developmental trajectory of digit DI, CIII into DII, and CIV into DIII. This hypothesis reconciles the findings of comparative anatomy, which show that the remaining three digits of birds are digits DI, DII, and DIII and the embryological data that demonstrate that these digits develop from condensations CII, CIII, and CIV.
We are not aware of any other case in which such a conflict between a developmental and a functional constraint in digit reduction existed. During the evolution of limbless forms, such as in snakes and other limbless lizards, for example, most functional constraints are relaxed, so reduction simply follows the pathway dictated by the developmental system. In the case of digit reduction in cursorial forms such as perissodactyls and artiodactyls, functional constraints tend not to be in conflict with development. For instance, in horses, the functionally most important digit is digit III, and digits II and IV are maintained only as rudimentary splints. Thus, loss of condensation CI and CV can proceed without interfering with the function of digit III.
Conclusions
Given the dissociation between digit and condensation identities in Alligator and Kiwi hands, the embryological and paleontological data cannot truly be said to be in conflict. These data, together with a propensity to homeotic transformation induced by CI loss in archosaur hands, inspired us to propose the Frame Shift Hypothesis, in which the developmental properties responsible for fingers DI—DIII are shifted onto condensations CII–CIV.
Novelties arising early in a life cycle can have profound influences on later development, which may explain how shared developmental and genetic pathways can so often succeed at providing congruent patterns of synapomorphy (56). Nevertheless, because evolutionary novelties can arise at any point in ontogeny (57), care should be taken to identify all of the developmental modules to which the homology relation might be applied, even if, at first glance, we seem to be dealing with a single “character.” Only in this way can one hope to avoid mistaking the homology of developmental precursors for the homology of the adult characters themselves.
Acknowledgments
G.P.W. dedicates this paper to his brother, Professor Wolfgang Wagner, who was his first intellectual mentor, on the occasion of his 50th birthday. We are grateful for comments on earlier versions of this manuscript by Chi-hua Chiu, Julia Clarke, Alan Gishlick, Kevin Padian, Kevin de Queiroz, and Elisabeth Vrba. We thank Anne Burke for sharing her manuscript on bird hand development with us before publication, Mark Norell for showing us an undescribed hand of Velociraptor, and Gerd Müller for discussing his experimental Alligator specimen with us. We greatly appreciate the original artwork by Lynn Barretti and the computer graphics and phylogenetic analyses done by Alan Gishlick. We also want to thank the staffs of the Yale Peabody Museum of Natural History, The Humboldt Museum (Berlin), the Jura Museum (Eichstätt, Germany), and the Museum of Comparative Zoology (Harvard University) for access to specimens in their care. The financial support through National Science Foundation Grants IBN-9507466 and IBN-9630567 to G.P.W. and BSR-87-09455 as well as a travel grant from the In-house Research Fund of The California Academy of Sciences to J.A.G. is gratefully appreciated.
Footnotes
A Commentary on this article begins on page 4740.
References
- 1.Burke A C, Feduccia A. Science. 1997;278:666–668. [Google Scholar]
- 2.Gauthier J. Mem California Acad Sci. 1986;8:1–55. [Google Scholar]
- 3.Meckel J F. System der vergleichenden Anatomie. Halle, Germany: Rengersche Buchhandlung; 1821. [Google Scholar]
- 4.Owen R. In: The Cyclopaedia of Anatomy and Physiology. Todd R B, editor. Vol. 1. Gilbert and Pipen, London: Sherwood; 1836. pp. 265–358. [Google Scholar]
- 5.Hinchliffe J R, Hecht M. Evol Biol. 1984;20:21–37. [Google Scholar]
- 6.Shubin N H. In: Homology: The Hierarchical Basis of Comparative Biology. Hall B K, editor. San Diego: Academic; 1994. pp. 249–271. [Google Scholar]
- 7.Heilmann G. The Origin of Birds. New York: Dover; 1927. [Google Scholar]
- 8.Lebedev O A. Priroda. 1985;11:26–36. [Google Scholar]
- 9.Baur, G. (1885) Science355. [DOI] [PubMed]
- 10.Parker W K. Philos Trans R Soc London B. 1891;13:45–83. [Google Scholar]
- 11.Sereno P C. J Vert Paleontol. 1993;13:385–399. [Google Scholar]
- 12.Ostrom J H. Bull Peabody Museum Nat History (Yale Univ) 1969;30:1–165. [Google Scholar]
- 13.Ostrom J. Biol J Linn Soc. 1976;8:91–182. [Google Scholar]
- 14.Galton P M. Arnoldia (Rhodesia) 1971;5:1–8. [Google Scholar]
- 15.Stephan B. In: The Beginnings of Birds. Hecht M K, Ostrom J H, Viohl G, Wellnhofer P, editors. Eichstätt, Germany: Freunde des Jura-Museums Eichstätt; 1985. pp. 261–265. [Google Scholar]
- 16.Bakker R, Galton P. Nature (London) 1974;248:169–172. [Google Scholar]
- 17.Baird D. In: Aspects of Vertebrate History: Essays in Honor of Edwin Harris Colbert. Jacobs L, editor. Flagstaff, AZ.: Museum of N. Arizona Press; 1980. [Google Scholar]
- 18.Bellairs A D A, Jenkin C R. In: Biology and Comparative Physiology of Birds. Marshall A J, editor. London: Academic; 1960. pp. 241–300. [Google Scholar]
- 19.Morse, E. (1872) Ann. Lyceum Nat. History (New York).
- 20.Hinchliffe J R. In: Vertebrate Limb and Somite Morphogenesis. Hinchliffe J R, Balls M, editors. Cambridge, U.K.: Cambridge Univ. Press; 1977. pp. 293–309. [Google Scholar]
- 21.Hinchliffe J R. In: The Beginnings of Birds. Hecht M K, Ostrom J H, Viohl G, Wellnhofer P, editors. Eichstätt, Germany: Freunde des Jura-Museums Eichstätt; 1985. pp. 141–147. [Google Scholar]
- 22.Shubin N H, Alberch P. Evol Biol. 1986;20:319–387. [Google Scholar]
- 23.Müller G B, Alberch P. J Morphol. 1990;203:151–164. doi: 10.1002/jmor.1052030204. [DOI] [PubMed] [Google Scholar]
- 24.Alberch P, Gale E A. J Embryol Exp Morphol. 1983;76:177–197. [PubMed] [Google Scholar]
- 25.Alberch P, Gale E A. Evolution (Lawrence, Kans) 1985;39:8–23. doi: 10.1111/j.1558-5646.1985.tb04076.x. [DOI] [PubMed] [Google Scholar]
- 26.Burke A C, Alberch P. J Morphol. 1985;186:119–131. doi: 10.1002/jmor.1051860111. [DOI] [PubMed] [Google Scholar]
- 27.Müller G B. In: Die Evolution der Evolutionstheorie. Wieser W, editor. Heidelberg: Spektrum Akademischer Verlag; 1994. pp. 155–193. [Google Scholar]
- 28.Gegenbaur C. Arch Anat Phys und wissenschaftliche Med. 1863;1863:450–472. [Google Scholar]
- 29.Norell M A, Makovicky P, Clark J M. Nature (London) 1997;389:447. [Google Scholar]
- 30.Chen P, Dong Z, Zhen S. Nature (London) 1998;391:147–152. [Google Scholar]
- 31.Ji Q, Currie P J, Norell M A, Ji S-A. Nature (London) 1998;393:753–761. [Google Scholar]
- 32.Holtz T R. J Paleontol. 1994;68:1100–1117. [Google Scholar]
- 33.Makovicky P J, Sues H D. J Vert Paleontol. 1997;17:62A. [Google Scholar]
- 34.Sereno P C. Annu Rev Earth Planet Sci. 1997;25:435–489. [Google Scholar]
- 35.Chiappe L M, Norell M A, Clark J M. Mem Queensland Museum. 1996;39:557–582. [Google Scholar]
- 36.Fedduccia A. The Origin and Evolution of Birds. New Haven, CT: Yale Univ. Press; 1996. [Google Scholar]
- 37.Hall B K. Evolutionary Developmental Biology. New York: Chapman & Hall; 1992. [Google Scholar]
- 38.Spemann H. In: Allgemeine Biologie. Chun C, Johannsen W, editors. Vol. 3. Leipzig, Germany: Teubner; 1915. pp. 63–86. [Google Scholar]
- 39.DeBeer G R. Homology: An Unsolved Problem. London: Oxford Univ. Press; 1971. [Google Scholar]
- 40.Roth V L. In: Ontogeny and Systematics. Humphries C J, editor. New York: Columbia Univ. Press; 1988. pp. 1–26. [Google Scholar]
- 41.Wagner G P. In: Homology: The Hierarchical Basis of Comparative Biology. Hall B K, editor. San Diego: Academic; 1994. pp. 273–299. [Google Scholar]
- 42.Wagner G P, Misof B Y. J Evol Biol. 1993;6:449–455. [Google Scholar]
- 43.Tabin C J. Development (Cambridge, UK) 1992;116:289–296. doi: 10.1242/dev.116.2.289. [DOI] [PubMed] [Google Scholar]
- 44.Nüsslein-Volhard C, Wieschaus E. Nature (London) 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 45.Burke A C, Nelson C E, Morgan B A, Tabin C. Development (Cambridge, UK) 1995;121:333–346. doi: 10.1242/dev.121.2.333. [DOI] [PubMed] [Google Scholar]
- 46.Johnson R L, Tabin C J. Cell. 1997;90:979–990. doi: 10.1016/s0092-8674(00)80364-5. [DOI] [PubMed] [Google Scholar]
- 47.Ng J K, Tamura K, Büscher D, Izpisúa-Belmonte J C. Curr Top Dev Biol. 1999;41:37–66. doi: 10.1016/s0070-2153(08)60269-0. [DOI] [PubMed] [Google Scholar]
- 48.Capdevila J, Izpisúa-Belmonte J C. In: The Character Concept in Evolutionary Biology. Wagner G P, editor. San Diego: Academic; 1999. , in press. [Google Scholar]
- 49.Zákány J, Fromental-Ramain C, Warot X, Duboule D. Proc Natl Acad Sci USA. 1997;94:13695–13700. doi: 10.1073/pnas.94.25.13695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fromental-Ramain C, Warot X, Messadecq N, LeMeur M, Dollé P, Chambon P. Development (Cambridge, UK) 1996;122:2997–3011. doi: 10.1242/dev.122.10.2997. [DOI] [PubMed] [Google Scholar]
- 51.Rijli F M, Chambon P. Curr Opin Genet Dev. 1997;7:481–487. doi: 10.1016/s0959-437x(97)80074-3. [DOI] [PubMed] [Google Scholar]
- 52.Duboule D. Science. 1994;266:575–576. doi: 10.1126/science.7939709. [DOI] [PubMed] [Google Scholar]
- 53.Hall B K, Miyake T. Int J Dev Biol. 1995;39:881–893. [PubMed] [Google Scholar]
- 54.Morgan B A, Izpisúa-Belmonte J-C, Duboule D, Tabin C J. Nature (London) 1992;358:236–239. doi: 10.1038/358236a0. [DOI] [PubMed] [Google Scholar]
- 55.Parker T J. Philos Trans R Soc London. 1891;181:25–134. [Google Scholar]
- 56.Queiroz K D. Syst Zool. 1985;34:280–299. [Google Scholar]
- 57.Gauthier J, Kluge A G, Rowe T. Cladistics. 1988;4:105–209. doi: 10.1111/j.1096-0031.1988.tb00514.x. [DOI] [PubMed] [Google Scholar]
- 58.Sereno P. J Vert Paleontol. 1993;13:425–450. [Google Scholar]
- 59.Colbert E H. Museum N Arizona Bull. 1989;57:1–160. [Google Scholar]