Abstract
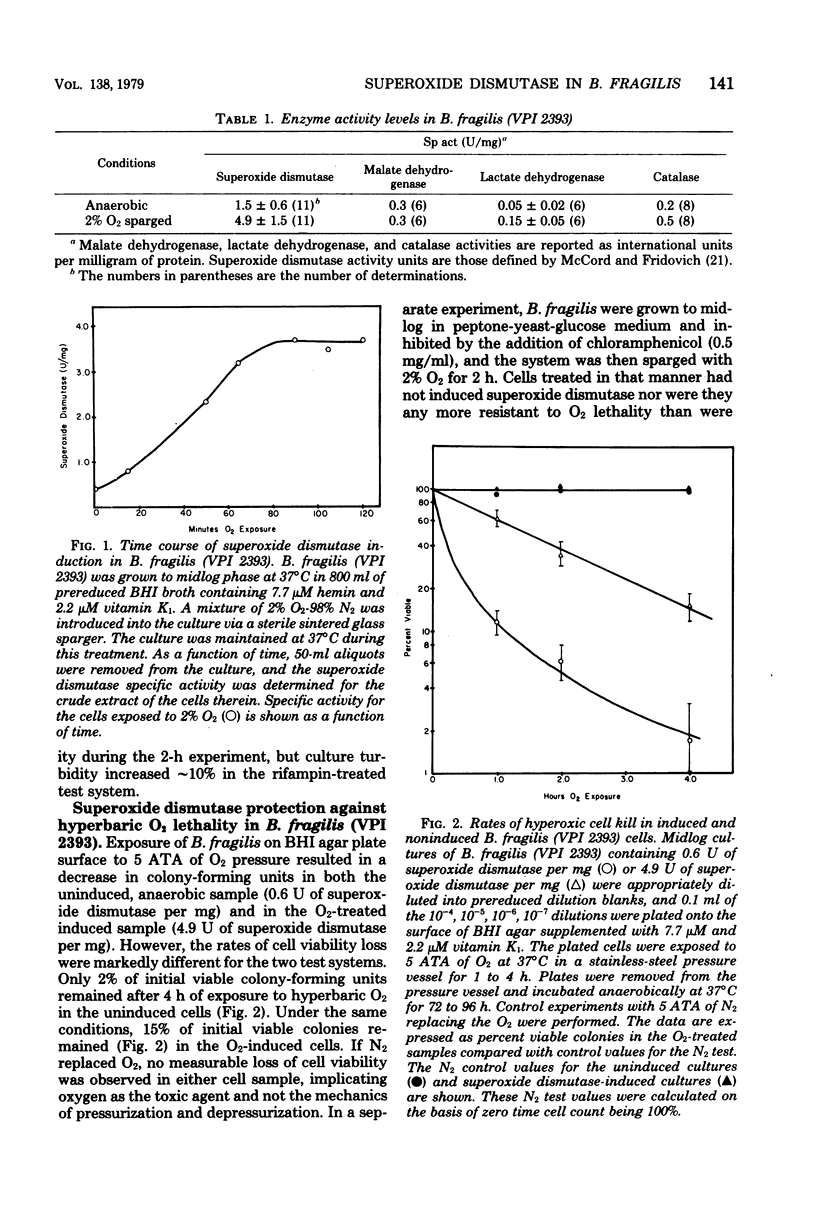
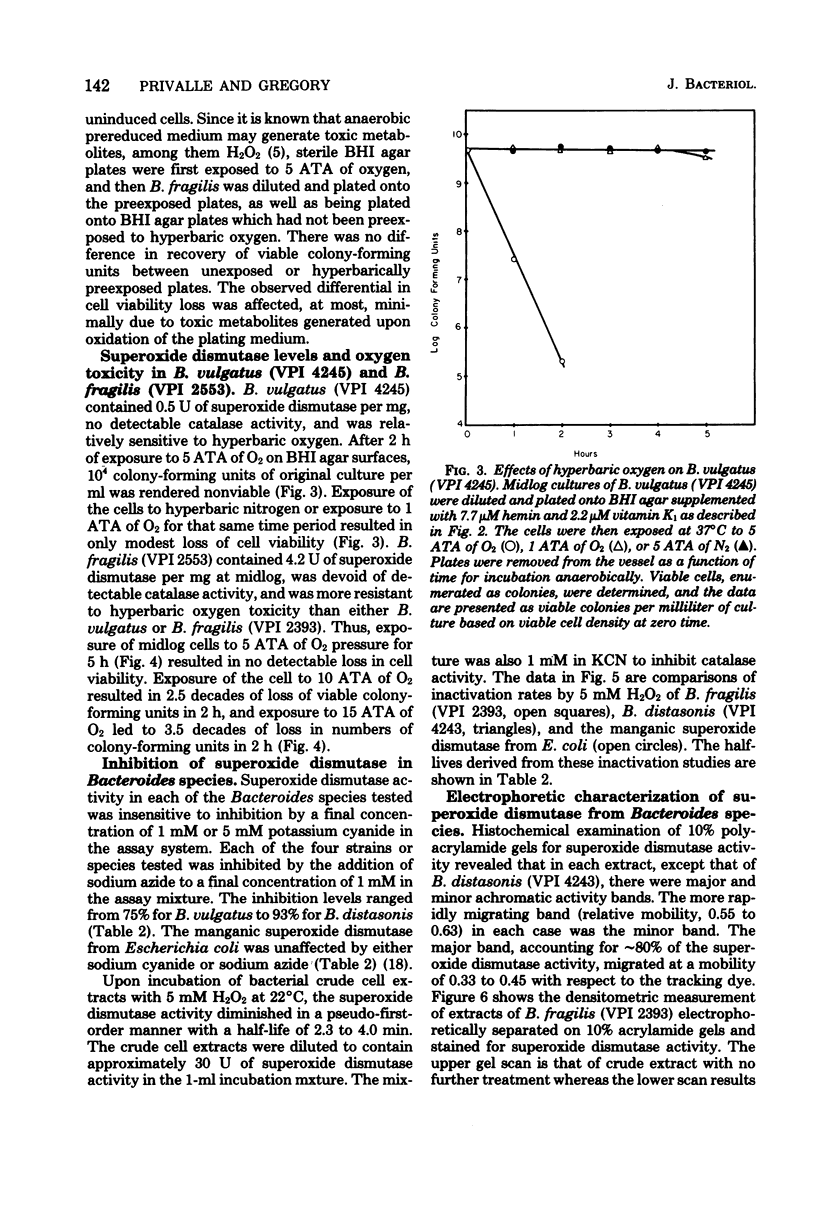
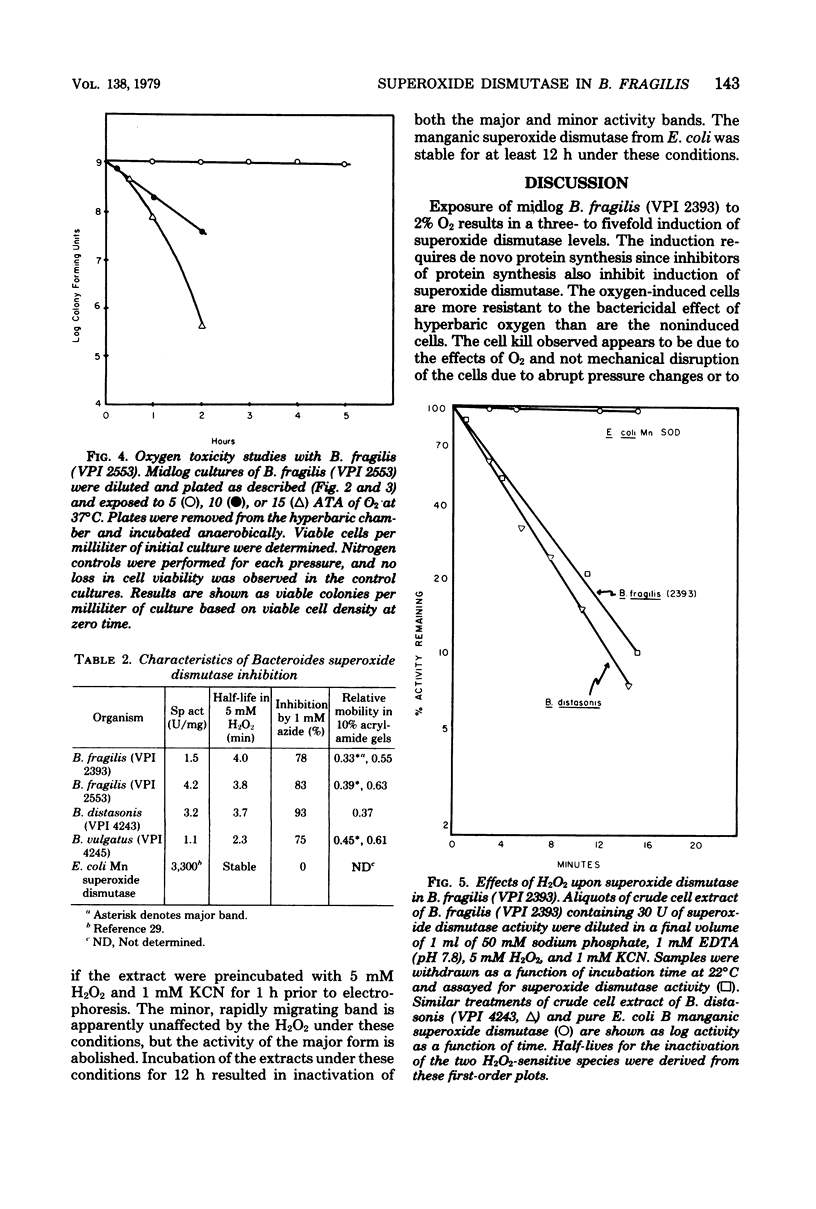
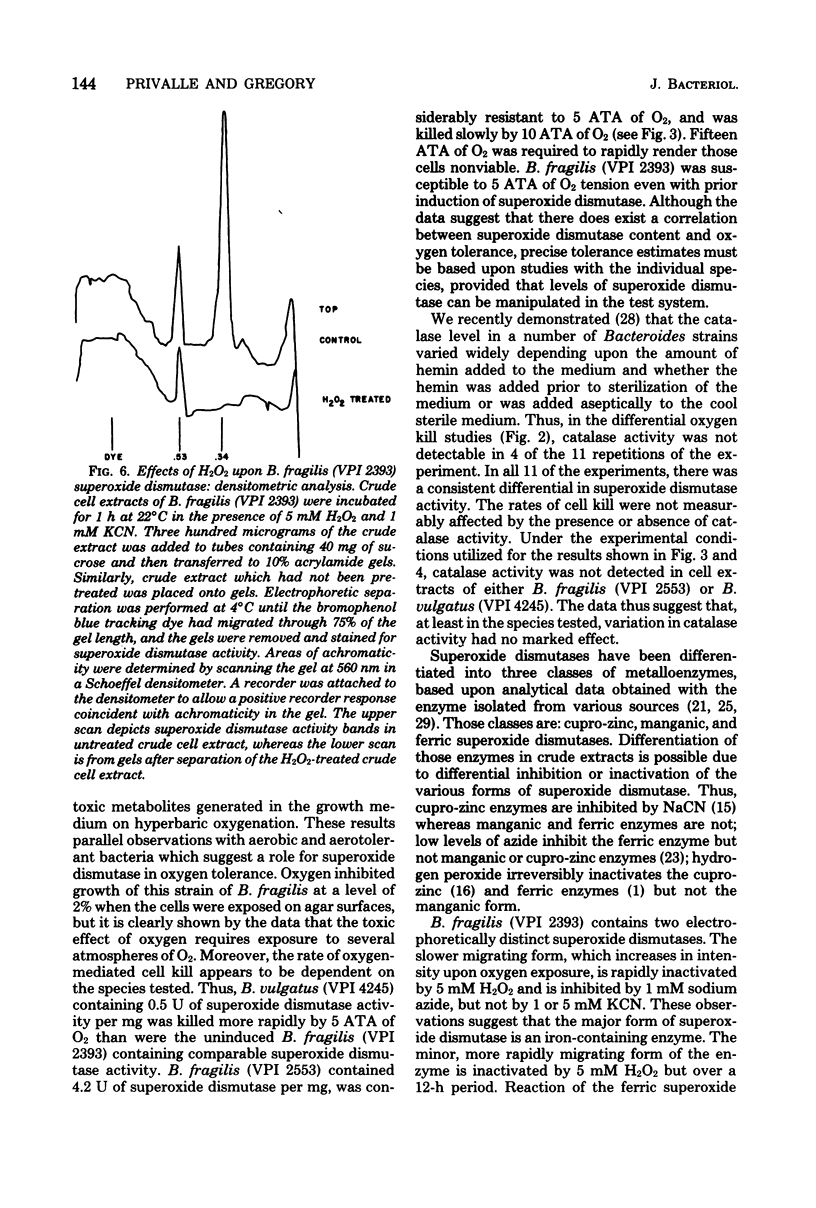
Exposure of midlog Bacteroides fragils (VPI 2393) to 2% O2-98% N2 caused a three- to fivefold increase in superoxide dismutase specific activity within the cells. The increase in specific activity was completed within 90 min after exposure to oxygen and was dependent upon protein synthesis. Cells containing the higher superoxide dismutase level were more resistant to the effects of 5 atm of oxygen tension than were cells containing the lower level of superoxide dismutase but were equally resistant to 5 atm of nitrogen tension. Similar results were observed upon comparing viability experiments with B. fragilis and B. vulgatus. Superoxide dismutase activity in sonic extracts of B. fragilis was rapidly inactivated by exposure to 5 mM H2O2 and was inhibited by 1 mM NaN3 but not 5 mM NaCN. The inhibition pattern is identical to the pattern demonstrated for the purified iron-containing enzyme from Escherichia coli B and suggests that the superoxide dismutase in B. fragilis is an iron enzyme.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asada K., Yoshikawa K., Takahashi M., Maeda Y., Enmanji K. Superoxide dismutases from a blue-green alga, Plectonema boryanum. J Biol Chem. 1975 Apr 25;250(8):2801–2807. [PubMed] [Google Scholar]
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Nyberg G., Wrethén J. Hydrogen peroxide and superoxide radical formation in anaerobic broth media exposed to atmospheric oxygen. Appl Environ Microbiol. 1978 Aug;36(2):223–229. doi: 10.1128/aem.36.2.223-229.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Wrethén J., Beckman G. Superoxide dismutase in Bacteroides fragilis and related Bacteroides species. J Clin Microbiol. 1977 Sep;6(3):280–284. doi: 10.1128/jcm.6.3.280-284.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Annu Rev Biochem. 1975;44:147–159. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Gregory E. M. Chemical modification of bovine heart mitochondrial malate dehydrogenase. Selective modification of cysteine and histidine. J Biol Chem. 1975 Jul 25;250(14):5470–5474. [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Induction of superoxide dismutase by molecular oxygen. J Bacteriol. 1973 May;114(2):543–548. doi: 10.1128/jb.114.2.543-548.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Oxygen toxicity and the superoxide dismutase. J Bacteriol. 1973 Jun;114(3):1193–1197. doi: 10.1128/jb.114.3.1193-1197.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Goscin S. A., Fridovich I. Superoxide dismutase and oxygen toxicity in a eukaryote. J Bacteriol. 1974 Feb;117(2):456–460. doi: 10.1128/jb.117.2.456-460.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Moore W. E., Holdeman L. V. Superoxide dismutase in anaerobes: survey. Appl Environ Microbiol. 1978 May;35(5):988–991. doi: 10.1128/aem.35.5.988-991.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffner P. H., Coleman J. E. Cu (II)-carbon bonding in cyanide complexes of copper enzymes. 13C splitting of the Cu(II) electron spin resonance. J Biol Chem. 1973 Oct 10;248(19):6626–6629. [PubMed] [Google Scholar]
- Hodgson E. K., Fridovich I. The interaction of bovine erythrocyte superoxide dismutase with hydrogen peroxide: inactivation of the enzyme. Biochemistry. 1975 Dec 2;14(24):5294–5299. doi: 10.1021/bi00695a010. [DOI] [PubMed] [Google Scholar]
- Keele B. B., Jr, McCord J. M., Fridovich I. Superoxide dismutase from escherichia coli B. A new manganese-containing enzyme. J Biol Chem. 1970 Nov 25;245(22):6176–6181. [PubMed] [Google Scholar]
- Koch A. L. Turbidity measurements of bacterial cultures in some available commercial instruments. Anal Biochem. 1970 Nov;38(1):252–259. doi: 10.1016/0003-2697(70)90174-0. [DOI] [PubMed] [Google Scholar]
- Loesche W. J. Oxygen sensitivity of various anaerobic bacteria. Appl Microbiol. 1969 Nov;18(5):723–727. doi: 10.1128/am.18.5.723-727.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- McCord J. M., Keele B. B., Jr, Fridovich I. An enzyme-based theory of obligate anaerobiosis: the physiological function of superoxide dismutase. Proc Natl Acad Sci U S A. 1971 May;68(5):1024–1027. doi: 10.1073/pnas.68.5.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. Inhibition of superoxide dismutases by azide. Arch Biochem Biophys. 1978 Aug;189(2):317–322. doi: 10.1016/0003-9861(78)90218-7. [DOI] [PubMed] [Google Scholar]
- Tally F. P., Goldin B. R., Jacobus N. V., Gorbach S. L. Superoxide dismutase in anaerobic bacteria of clinical significance. Infect Immun. 1977 Apr;16(1):20–25. doi: 10.1128/iai.16.1.20-25.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance P. G., Keele B. B., Jr, Rajagopalan K. V. Superoxide dismutase from Streptococcus mutans. Isolation and characterization of two forms of the enzyme. J Biol Chem. 1972 Aug 10;247(15):4782–4786. [PubMed] [Google Scholar]
- Wehrli W., Knüsel F., Schmid K., Staehelin M. Interaction of rifamycin with bacterial RNA polymerase. Proc Natl Acad Sci U S A. 1968 Oct;61(2):667–673. doi: 10.1073/pnas.61.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins T. D., Wagner D. L., Veltri B. J., Jr, Gregory E. M. Factors affecting production of catalase by Bacteroides. J Clin Microbiol. 1978 Nov;8(5):553–557. doi: 10.1128/jcm.8.5.553-557.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost F. J., Jr, Fridovich I. An iron-containing superoxide dismutase from Escherichia coli. J Biol Chem. 1973 Jul 25;248(14):4905–4908. [PubMed] [Google Scholar]



