Abstract
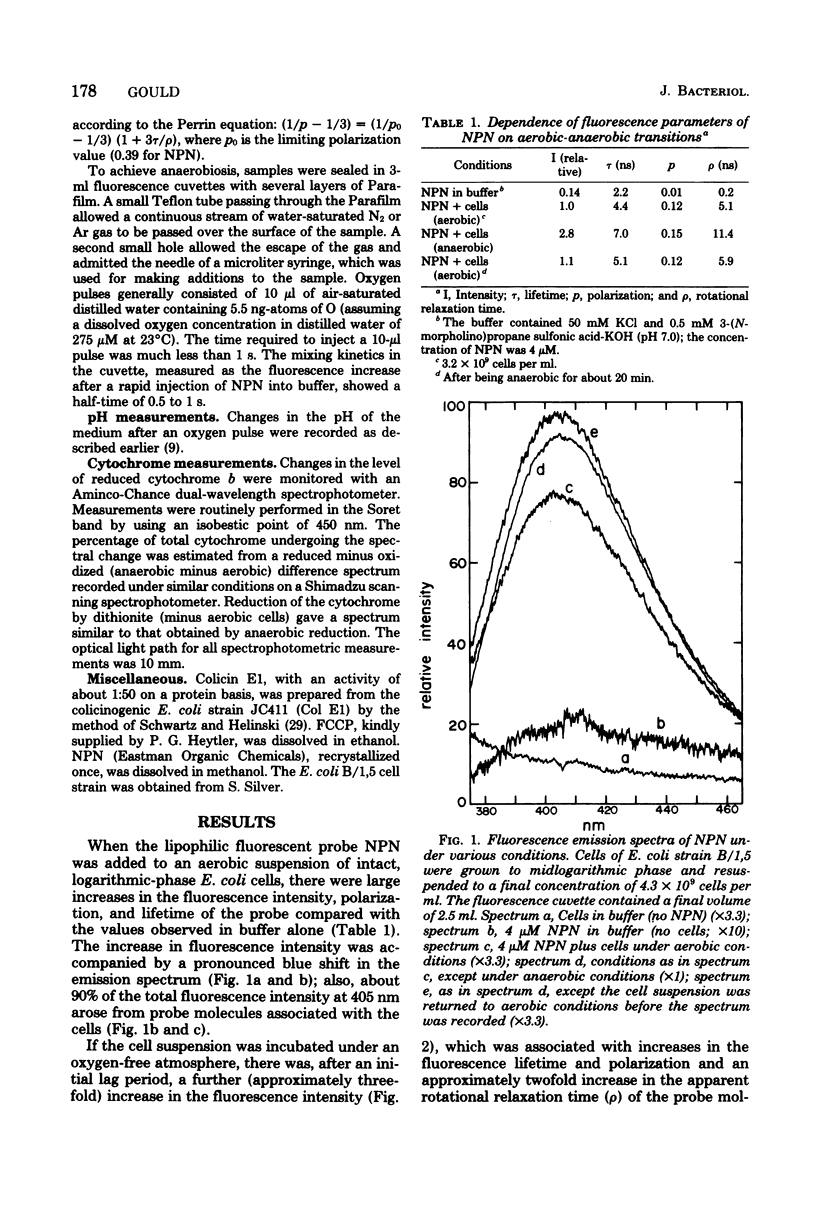
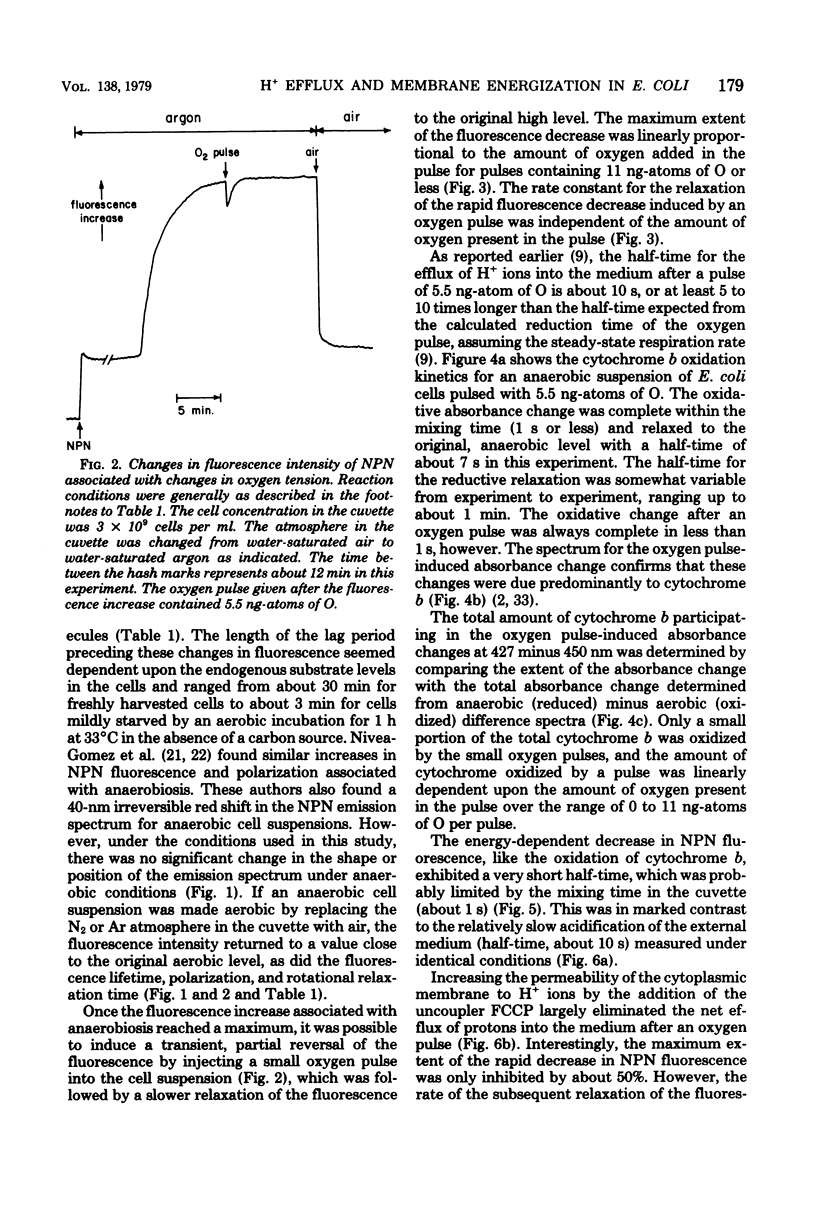
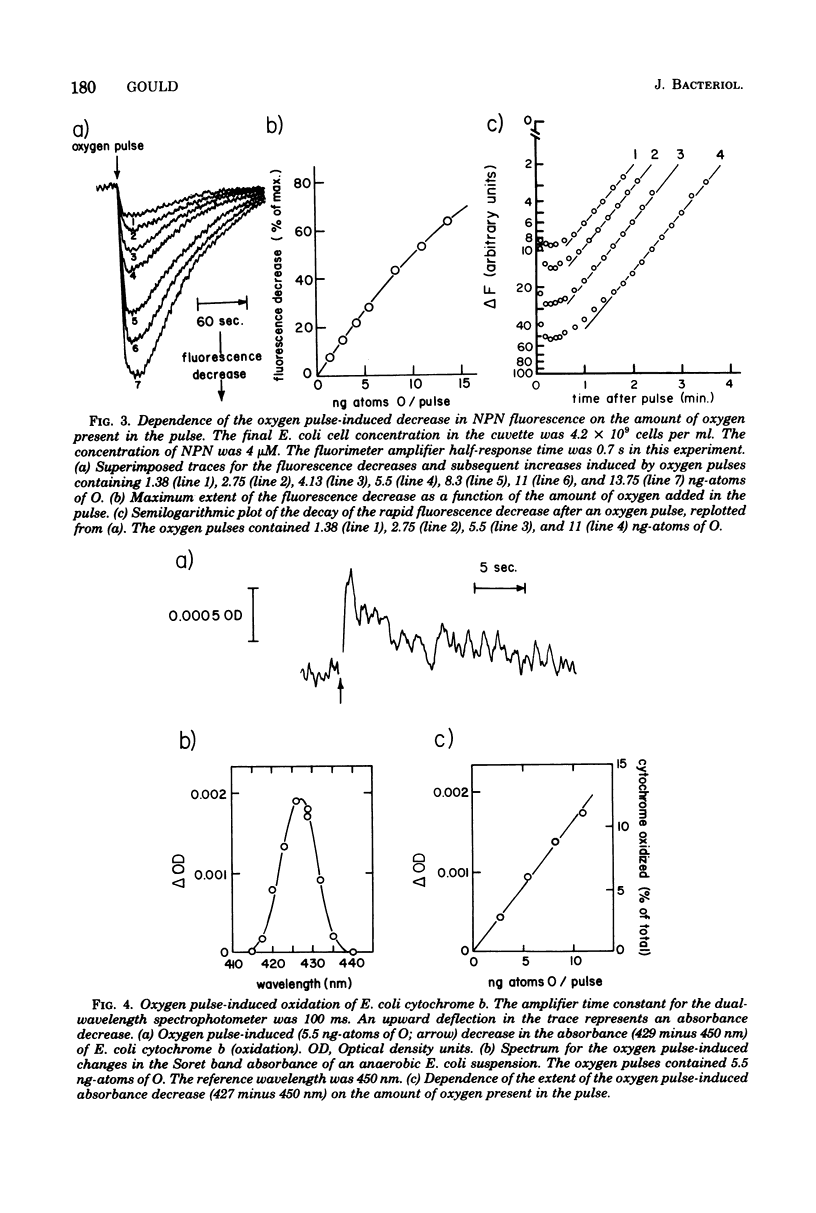
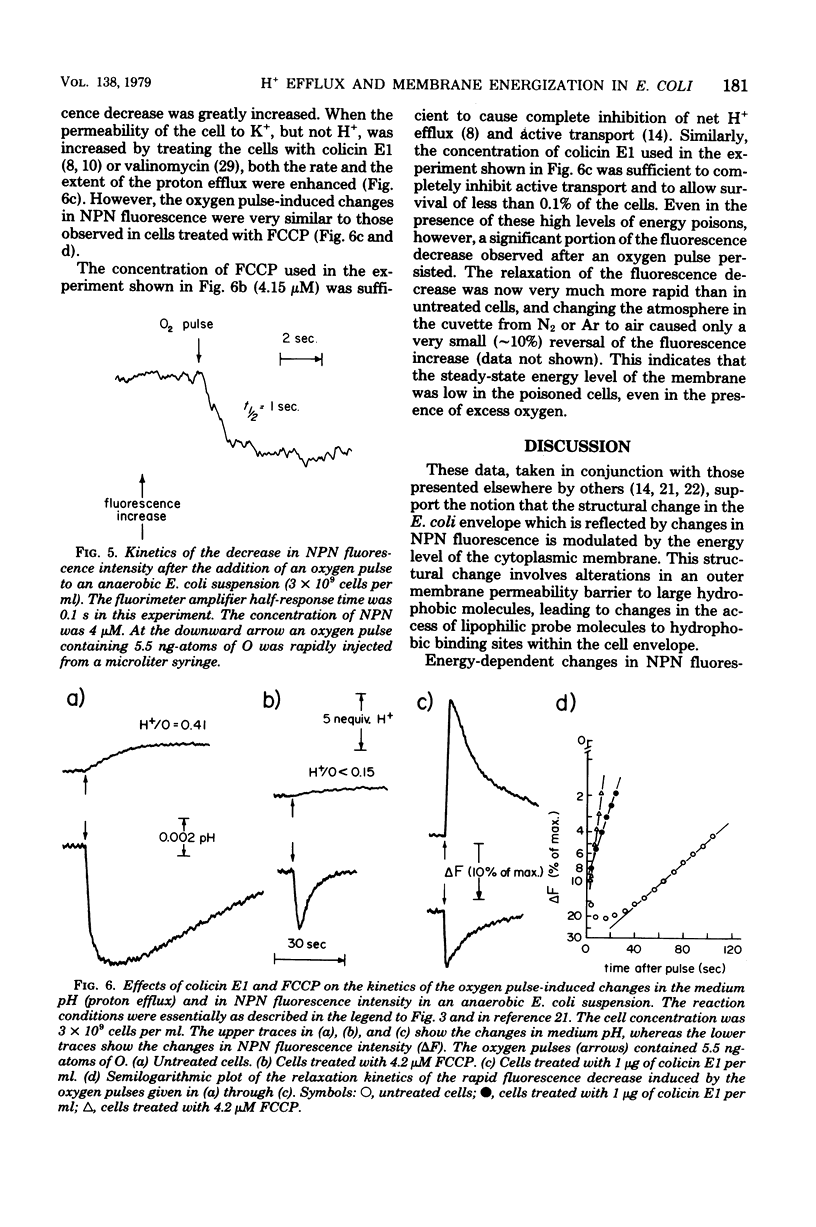
The kinetics of respiration-dependent proton efflux and membrane energization have been studied in intact cells of logarithmic-phase Escherichia coli. Parallel measurements of the rate and extent of proton efflux into the external medium (half-time, about 10 s; ratio of H+ to O, about 0.5) and the oxidation of E. coli cytochrome b (half-time, ≤1 s; about 6% oxidized) after a pulse of 5.5 ng-atoms of O indicate that the rate of proton efflux is at least 10 times slower than expected from the time required for the cells to reduce the oxygen added in the pulse. The kinetics of formation and dissipation of the transmembrane electric potential (δψ) after an O2 pulse were estimated from changes in the fluorescence properties of the cell envelope-bound probe N-phenyl-1-naphthylamine. Under anaerobic conditions, a small pulse of oxygen induced a rapid (half-time, ≤1 s) partial decrease in the fluorescence intensity of the probe, followed by a slower relaxation of the fluorescence change to the original intensity. The extent of the initial rapid decrease was linearly dependent upon the amount of oxygen added in the pulse (0 to 11 ng-atoms of O per pulse), whereas the rate of the subsequent relaxation was accelerated by the uncoupler p-trifluoromethoxycarbonylcyanidephenylhydrazone and the K+ ionophore colicin E1. This suggests that the initial fluorescence decrease after an O2 pulse reflects the energization of the membrane, whereas the relaxation of the fluorescence decrease reflects the subsequent deenergization of the membrane arising from counterion redistributions. The fact that the efflux of H+ into the external medium after an O2 pulse was inefficient and much slower (half-time, about 10 s) than the reduction of the added O2 (half-time, ≤1 s) and the energization of the membrane (half-time ≤1 s) suggests that some of the protons translocated across the cytoplasmic membrane during a brief pulse of respiratory activity are accumulated in a region of the cell which is not in rapid equilibrium with the external medium.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anraku Y. The reduction and restoration of galactose transport in osmotically shocked cells of Escherichia coli. J Biol Chem. 1967 Mar 10;242(5):793–800. [PubMed] [Google Scholar]
- Cox G. B., Newton N. A., Gibson F., Snoswell A. M., Hamilton J. A. The function of ubiquinone in Escherichia coli. Biochem J. 1970 Apr;117(3):551–562. doi: 10.1042/bj1170551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer W. A., Phillips S. K., Keenan T. W. On the role of membrane phase in the transmission mechanism of colicin E1. Biochemistry. 1973 Mar 13;12(6):1177–1181. doi: 10.1021/bi00730a025. [DOI] [PubMed] [Google Scholar]
- Cramer W. A., Phillips S. K. Response of an Escherichia coli-bound fluorescent probe to colicin E1. J Bacteriol. 1970 Nov;104(2):819–825. doi: 10.1128/jb.104.2.819-825.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer W. A., Postma P. W., Helgerson S. L. An evaluation of N-phenyl-1-naphthylamine as a probe of membrane energy state in Escherichia coli. Biochim Biophys Acta. 1976 Dec 6;449(3):401–411. doi: 10.1016/0005-2728(76)90151-1. [DOI] [PubMed] [Google Scholar]
- Dyar M. T., Ordal E. J. Electrokinetic Studies on Bacterial Surfaces: I. Effects of Surface-active Agents on Electrophoretic Mobilities of Bacteria. J Bacteriol. 1946 Feb;51(2):149–167. [PMC free article] [PubMed] [Google Scholar]
- Gould J. M., Cramer W. A. Relationship between oxygen-induced proton efflux and membrane energization in cells of Escherichia coli. J Biol Chem. 1977 Aug 25;252(16):5875–5882. [PubMed] [Google Scholar]
- Gould J. M., Cramer W. A. Studies on the depolarization of the Escherichia coli cell membrane by colicin E1. J Biol Chem. 1977 Aug 10;252(15):5491–5497. [PubMed] [Google Scholar]
- Gould J. M., Cramer W. A., van Thienen G. The effect of colicin E1 on proton extrusion and the H+/0 ration in Escherichia coli. Biochem Biophys Res Commun. 1976 Oct 18;72(4):1519–1525. doi: 10.1016/s0006-291x(76)80186-6. [DOI] [PubMed] [Google Scholar]
- Gould J. M. The kinetics of oxygen-induced proton efflux and membrane energization in Escherichia coli. Prog Clin Biol Res. 1978;22:567–578. [PubMed] [Google Scholar]
- Griniuviene B., Chmieliauskaite V., Grinius L. Energy-linked transport of permeant ions in Escherichia coli cells: evidence for membrane potential generation by proton-pump. Biochem Biophys Res Commun. 1974 Jan;56(1):206–213. doi: 10.1016/s0006-291x(74)80335-9. [DOI] [PubMed] [Google Scholar]
- Helgerson S. L., Cramer W. A. Changes in Escherichia coli cell envelope structure and the sites of fluorescence probe binding caused by carbonyl cyanide p-trifluoromethoxyphenylhydrazone. Biochemistry. 1977 Sep 6;16(18):4109–4117. doi: 10.1021/bi00637a026. [DOI] [PubMed] [Google Scholar]
- Helgerson S. L., Cramer W. A., Harris J. M., Lytle F. E. Evidence for a microviscosity increase in the Escherichia coli cell envelope caused by colicin E1. Biochemistry. 1974 Jul 16;13(15):3057–3061. doi: 10.1021/bi00712a010. [DOI] [PubMed] [Google Scholar]
- Hirata H., Altendorf K., Harold F. M. Role of an electrical potential in the coupling of metabolic energy to active transport by membrane vesicles of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1804–1808. doi: 10.1073/pnas.70.6.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQUILLEN K. The bacterial surface. I. Effect of cetyl-trimethyl-ammonium bromide on the electrophoretic mobility of certain gram-positive bacteria. Biochim Biophys Acta. 1950 Jun;5(3/4):463–471. doi: 10.1016/0006-3002(50)90192-2. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Vectorial chemiosmotic processes. Annu Rev Biochem. 1977;46:996–1005. doi: 10.1146/annurev.bi.46.070177.005024. [DOI] [PubMed] [Google Scholar]
- Nieva-Gomez D., Gennis R. B. Affinity of intact Escherichia coli for hydrophobic membrane probes is a function of the physiological state of the cells. Proc Natl Acad Sci U S A. 1977 May;74(5):1811–1815. doi: 10.1073/pnas.74.5.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieva-Gomez D., Konisky J. Membrane changes in Escherichia coli induced by colicin Ia and agents known to disrupt energy transduction. Biochemistry. 1976 Jun 29;15(13):2747–2753. doi: 10.1021/bi00658a006. [DOI] [PubMed] [Google Scholar]
- Ort D. R., Dilley R. A., Good N. E. Photophosphorylation as a function of illumination time. II. Effects of permeant buffers. Biochim Biophys Acta. 1976 Oct 13;449(1):108–124. doi: 10.1016/0005-2728(76)90011-6. [DOI] [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Rottenberg H. The proton electrochemical gradient in Escherichia coli cells. Eur J Biochem. 1976 Apr 1;63(2):533–541. doi: 10.1111/j.1432-1033.1976.tb10257.x. [DOI] [PubMed] [Google Scholar]
- Phillips S. K., Cramer W. A. Properties of the fluorescence probe response associated with the transmission mechanism of colicin E1. Biochemistry. 1973 Mar 13;12(6):1170–1176. doi: 10.1021/bi00730a024. [DOI] [PubMed] [Google Scholar]
- Rosen B. P. Beta-galactoside transport and proton movements in an adenosine triphosphatase-deficient mutant of Escherichia coli. Biochem Biophys Res Commun. 1973 Aug 21;53(4):1289–1296. doi: 10.1016/0006-291x(73)90605-0. [DOI] [PubMed] [Google Scholar]
- Scholes P., Mitchell P. Acid-base titration across the plasma membrane of Micrococcus denitrificans: factors affecting the effective proton conductance and the respiratory rate. J Bioenerg. 1970 Jun;1(1):61–72. doi: 10.1007/BF01516089. [DOI] [PubMed] [Google Scholar]
- Schwartz S. A., Helinski D. R. Purification and characterization of colicin E1. J Biol Chem. 1971 Oct 25;246(20):6318–6327. [PubMed] [Google Scholar]
- Simoni R. D., Postma P. W. The energetics of bacterial active transport. Annu Rev Biochem. 1975;44:523–554. doi: 10.1146/annurev.bi.44.070175.002515. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Rauch B., Roseman S. Periplasmic space in Salmonella typhimurium and Escherichia coli. J Biol Chem. 1977 Nov 10;252(21):7850–7861. [PubMed] [Google Scholar]
- TABER H. W., MORRISON M. ELECTRON TRANSPORT IN STAPHYLOCOCCI. PROPERTIES OF A PARTICLE PREPARATION FROM EXPONENTIAL PHASE STAPHYLOCOCCUS AUREUS. Arch Biochem Biophys. 1964 May;105:367–379. doi: 10.1016/0003-9861(64)90021-9. [DOI] [PubMed] [Google Scholar]
- Waggoner A. S., Grinvald A. Mechanisms of rapid optical changes of potential sensitive dyes. Ann N Y Acad Sci. 1977 Dec 30;303:217–241. [PubMed] [Google Scholar]
- West I. C. Lactose transport coupled to proton movements in Escherichia coli. Biochem Biophys Res Commun. 1970 Nov 9;41(3):655–661. doi: 10.1016/0006-291x(70)90063-x. [DOI] [PubMed] [Google Scholar]
- West I. C., Mitchell P. Stoicheiometry of lactose-H+ symport across the plasma membrane of Escherichia coli. Biochem J. 1973 Mar;132(3):587–592. doi: 10.1042/bj1320587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West I. C., Wilson T. H. Galactoside transport dissociated from proton movement in mutants of Escherichia coli. Biochem Biophys Res Commun. 1973 Jan 23;50(2):551–558. doi: 10.1016/0006-291x(73)90875-9. [DOI] [PubMed] [Google Scholar]
- West I., Mitchell P. Proton-coupled beta-galactoside translocation in non-metabolizing Escherichia coli. J Bioenerg. 1972 Aug;3(5):445–462. doi: 10.1007/BF01516082. [DOI] [PubMed] [Google Scholar]
- Williams R. J. The multifarious couplings of energy transduction. Biochim Biophys Acta. 1978 Sep 21;505(1):1–44. doi: 10.1016/0304-4173(78)90007-1. [DOI] [PubMed] [Google Scholar]



