Abstract
Recently, hptc, a human gene homologous to the Drosophila segment polarity gene patched (ptc), has been implicated in the nevoid basal-cell carcinoma (BCC) syndrome, and somatic mutations of hptc also have been found in sporadic BCCs, the most frequent cancers found in the white population. We have analyzed the hptc gene, postulated to be a tumor suppressor gene, in 22 BCCs from patients with the hyperphotosensitive genodermatosis xeroderma pigmentosum (XP). Patients with XP are deficient in the repair of UV-induced DNA lesions and are characterized by their predisposition to cancers in sun-exposed skin. Analysis using PCR–single-strand conformation polymorphism of the hptc gene identified 19 alterations in 16 of 22 (73%) of the BCCs examined. Only two (11%) deletions of the hptc gene were found in XP BCCs compared with >30% rearrangement observed in non-XP sporadic BCCs, and 17 of 19 (89%) were base substitutions. Among the 17 base substitutions, 11 (65%) were CC → TT tandem mutations, and 4 (23%) were C → T substitutions, all targeted at bipyrimidine sites. Hence, a significantly higher number (15 of 19; 79%) of UV-specific alterations are seen in XP tumors, in contrast to non-XP sporadic BCCs. Interestingly, we have found that in 7 of 14 (50%) XP BCCs analyzed, both hptc and the tumor suppressor gene p53 are mutated. Not only have our data indicated the key role played by hptc in the development of BCCs, they also have substantiated the link between unrepaired UV-induced DNA lesions and skin carcinogenesis, as exemplified by the UV-specific alterations of different genes in the same tumors.
Keywords: hptc tumor suppressor gene, UV-specific mutations, DNA repair
Basal-cell carcinoma (BCC) is the most frequent skin cancer in the white population (1). BCCs mostly occur sporadically in relation to sun exposure, although their incidence is increased significantly in some rare genetic disorders. For instance, patients with either the nevoid BCC (NBCC) syndrome, also known as Gorlin’s syndrome (2), or the Bazex-Dupré-Christol syndrome (3) have numerous BCCs, together with characteristic developmental abnormalities. Patients with xeroderma pigmentosum (XP), a rare autosomal recessive syndrome characterized by hyperphotosensitivity, also are highly prone to cutaneous cancers, including BCCs.
Recently, the gene involved in the development of the NBCC syndrome has been identified as the human homologue of the Drosophila segment polarity gene patched (hptc; refs. 4 and 5). hptc maps to chromosome 9q22.3 and encodes a transmembrane glycoprotein that is the receptor for the hedgehog (HH) signaling protein (6, 7). The HPTC protein thus plays a key role in the regulation of the HH signaling pathway, whose main impact is on the control of cell differentiation and proliferation (8). Germ-line mutations of hptc are characteristic of the NBCC syndrome, and somatic mutations also have been found in sporadic BCCs occurring in the general population (9). Moreover, mutations of the hptc gene often are associated with loss of heterozygosity of the 9q chromosome, suggesting that hptc can behave as a tumor suppressor gene (4, 9).
The high incidence of epitheliomas that develop in sun-exposed areas of the skin of patients with XP (i.e., >4,000 times the incidence in the general population) has implicated the role of unrepaired UV-induced DNA lesions in skin carcinogenesis (10). Indeed, it has been shown that, because of the alteration of specific genes implicated in nucleotide excision repair, XP cells cannot eliminate UV-induced DNA lesions efficiently. Thus, the replication of unrepaired lesions can lead to the so-called “UV-specific” DNA mutations, which are mainly C → T transitions targeted at bipyrimidine sequences and CC → TT tandem mutations considered to be the veritable UV signature (11, 12). These UV-induced DNA mutations occur with much higher frequencies in UV-irradiated cultured XP cells compared with normal cells and in XP skin tumors compared with skin tumors from the normal population (13, 14). Thus, XP provides a very useful human model for studying the relationship between unrepaired UV-induced DNA lesions, mutations, and skin carcinogenesis. This model is best illustrated in recent molecular epidemiology studies (including those from our laboratory) that have identified higher levels of mutations in the ras oncogenes (13) and in the p53 tumor suppressor gene (14, 15) in XP carcinomas compared with the same genes in non-XP epidermal carcinomas. Moreover, analysis of the p53 mutation spectrum has shown clearly that the majority of p53 mutations are UV-specific in both XP and non-XP skin tumors, indicating the causative role played by sunlight in this carcinogenic process (15–17).
In this study, we analyzed the hptc gene in DNA isolated from XP BCCs, because it is considered to be a tumor suppressor gene that can be implicated in carcinogenesis through gene loss or misexpression and that also is involved in the development of BCCs. Our data have confirmed the presence of high levels of UV-induced mutations (C → T or CC → TT transitions) all located at bipyrimidine sites on the hptc gene. Moreover, in 7 of 14 (50%) BCCs from patients with XP, both hptc and p53 are mutated, indicating that these genes are targets for alterations in the multistep mechanisms involved in skin tumorigenesis. Our analysis of epitheliomas from repair-deficient patients with XP further strengthens the link between UV-induced DNA lesions, DNA repair, and skin carcinogenesis.
MATERIALS AND METHODS
Clinical Samples.
During surgical resections, 22 BCCs from 20 patients with XP (2 independent tumors were taken from 2 individual patients) were obtained. As it was not possible to carry out microdissection of tumor samples, the maximum amount possible of the surrounding normal tissue was removed. The debulked material was snap-frozen in liquid nitrogen and then stored at −80°C. Healthy skin from unexposed parts of the bodies of patients with XP (34 samples) or from the bodies of normal white individuals (8 samples) was obtained when possible, and primary skin fibroblast cultures were established in MEM (GIBCO/BRL) supplemented with 15% (vol/vol) FCS and 1% (vol/vol) antibiotics and fungicides.
The majority of the samples for biopsy were obtained from the Institut Gustave Roussy or from the Centre Hospitalo-Universitaire de Bab El Oued; the remaining samples were from different hospitals throughout France.
Isolation of DNA.
Tumor tissue finely ground in liquid nitrogen and tissue cultures collected as cell pellets were lysed in 50 mM Tris⋅HCl, pH 8.0/10 mM NaCl/10 mM EDTA/0.5% SDS/100 mg/ml proteinase K. After incubation at 37°C and two extractions with phenol/chloroform, the aqueous phase containing DNA was dialyzed once with TE (10 mM Tris⋅HCl, pH 8.0/1 mM EDTA) containing 1 M NaCl buffer, followed by a second dialysis with TE alone.
Single-Strand Conformation Polymorphism (SSCP) Analysis.
SSCP analysis of the 22 exons of the hptc gene was carried out by using primers described in previous studies (4, 18). PCRs were carried out in 25-μl volumes containing 100–200 ng of DNA, each dNTP at 100 μM, each primer at 0.2 μM, 1.5 units of Goldstar Taq DNA polymerase (Eurogentec, Angers, France), and 5 μCi of [α-33P]dATP (Amersham Pharmacia); PCRs were buffered in 75 mM Tris⋅HCl, pH 9/20 mM (NH4)2SO4/1.5 mM MgCl2/0.01% Tween 20. Cycling parameters were 2 min at 94°C followed by 35 cycles of 94°C for 30 s, 50–60°C for 30 s, and 72°C for 1 min (Gene Amp PCR System 9600, Perkin–Elmer). An extension period of 10 min at 72°C was added at the end of the program, and reactions were stopped by a 3-fold dilution in stop solution [95% (vol/vol) formamide/20 mM EDTA/0.05% bromophenol blue/0.05% xylene cyanole]. The PCR products were denatured for 5 min at 100°C and quick-chilled on ice. They were then analyzed in duplicate on 0.5× MDE gels (FMC) with and without 10% (vol/vol) glycerol and run at 6–10 W for 16–22 h at room temperature. After autoradiography, shifted bands were eluted from gels, amplified, and sequenced after purification. All samples were analyzed at least twice by SSCP to avoid polymerase errors and to confirm the presence of tumor-specific band shifts.
DNA Sequencing.
The SSCP variant bands were treated with shrimp alkaline phosphatase and exonuclease I and cycle-sequenced with the Thermosequenase kit (Amersham Pharmacia). The tumor DNAs that did not present mobility-shift changes in the SSCP analysis also were analyzed directly by cycle sequencing after PCR amplification of the hptc gene.
RESULTS
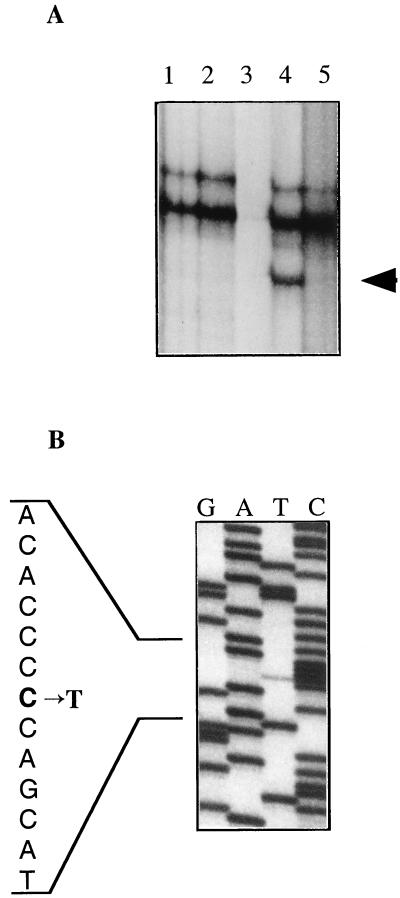
The majority of the tumors used in this study are from young patients with XP from North Africa, where the majority of patients with classic XP belong to the XP complementation group C in which the XPC gene is mutated. DNA from 22 BCCs obtained from patients with XP was screened for mutations in the hptc gene by PCR-SSCP analysis. All the exons were analyzed systematically at least twice by using primers homologous to intron sequences (4, 18), and all DNAs presenting a mobility shift (Fig. 1A) on the SSCP gel were sequenced. The 7 XP BCC tumor DNAs, which did not show band shifts on SSCP analysis, were analyzed further by direct sequencing after PCR amplification of the hptc gene, and one new mutation, a C1996T transition, was found in exon 13 of tumor DNA from patient M.J. (Fig. 1B). This DNA did not show a mobility shift in SSCP analysis (Fig. 1A, lane 1), and a very weak but clear mutated band is detected by direct sequence analysis (Fig. 1B). This probably is caused by a very heterogeneous populations of cells in our tumors that could not be subjected to microdissection before molecular analysis, as the majority of them were obtained directly from North Africa. Thus, in our study, 19 alterations, which spanned the whole hptc gene, were characterized in 16 of 22 (73%) BCCs, and 3 tumors presented 2 different mutations (Table 1). Moreover, hptc polymorphisms that had not been described previously were identified in our analysis.
Figure 1.
(A) SSCP analysis of amplified exon 13 DNA from various patients with XP. (Lane 1) BCC from patient M.J. (Lane 2) Squamous-cell carcinoma (SCC) from patient M.J. (Lane 3) Blank—no sample. (Lane 4) BCC from patient M.H. (Lane 5) Unexposed healthy skin from patient M.H. The presence of a shifted band (arrowhead, lane 4) compared with its absence in a wild-type sample (lane 5) indicates a modified sequence (see Table 1). (B) Direct sequence analysis of amplified exon 13 DNA of a BCC from patient M.J. that did not show mobility shift by SSCP analysis (A, lane 1). The C → T transition is seen as a weak but clear band compared with the wild-type allele sequence.
Table 1.
Mutations of the hptc gene in BCCs from patients with XP
| Patient | Localization | Exon | Mutation | Mutated codon | Effect on coding | DNA strand |
|---|---|---|---|---|---|---|
| A.M. (1) | nose | 3 | CC537TT | 183–184 | Leu–Gln → Leu–stop | NT |
| 6 | CC867TT | 293–294 | Asp–Arg → Asp–Cys | NT | ||
| K.L. | cheek | 6 | CC867TT | 293–294 | Asp–Arg → Asp–Cys | NT |
| M.C. | forehead | 8 | cAg1070cCg | 361 | Gln → Pro | T |
| Mo.H. (1) | nose | 11 | tTc1536tAc | 516 | Phe → Leu | NT |
| Am.S. | cheek | 13 | CC1974TT | 662–663 | Val–Gln → Val–stop | NT |
| 13 | cCa2093cTa | 702 | Pro → Leu | NT | ||
| M.J. | cheek | 13 | Ccc1996Tcc | 670 | Pro → Ser | NT |
| M.H. | ND | 13 | 2122del11 | — | frameshift | — |
| R.R. | forearm | 14 | CC2295TT | 769–770 | Thr–Arg → Thr–stop | NT |
| H.B. | eyelid | 14 | CC2295TT | 769–770 | Thr–Arg → Thr–stop | NT |
| 19 | CC3362TT | 1125 | Pro → Leu | NT | ||
| Am.T. | cheek | 14 | CC2295TT | 769–770 | Thr–Arg → Thr–stop | NT |
| Re.K. | cheek | 16 | CC2735AT | 916 | Pro → His | NT |
| A.H. | cheek | 16 | GG2766AA | 926–927 | Trp–Val → stop | T |
| Mo.H. (2) | cheek | 18 | 3237del G | — | frameshift | — |
| Ko.H. | ND | 19 | CC3308TT | 1107 | Arg → Val | NT |
| S.T. | ND | 21 | cCc3593cTc | 1202 | Pro → Leu | NT |
| A.M. (2) | nose | 22 | CC3803TT | 1272 | Pro → Leu | NT |
The patients from whom BCCs were taken are indicated by their initials. Numbers in brackets for patients A.M. and Mo.H. indicate different BBCs from these patients. The mutations are indicated in capitals on the coding strand, written 5′ to 3′, as described by Hahn et al. (4). Amino acids are numbered as described by Johnson et al. (5). The column entitled DNA strand gives the location of a Py–Py lesion on the transcribed strand (T) or the nontranscribed strand (NT) of the hptc gene. ND, not determined. All patients with XP, except M.H. and M.J., are from North Africa and, thus, probably belong to the complementation group C. M.J. is an XP variant.
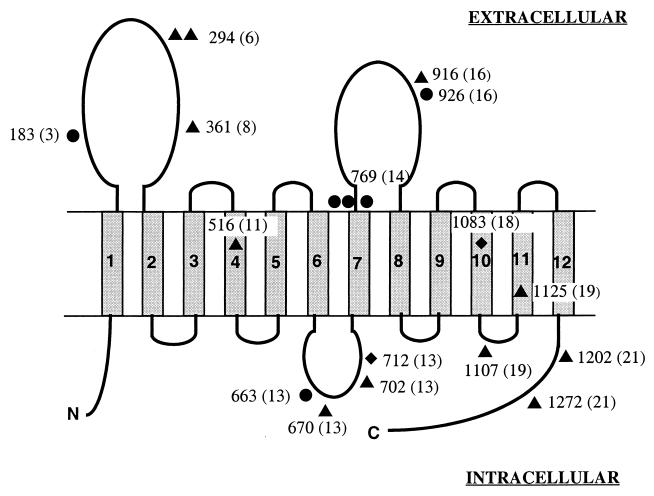
Of the 19 hptc alterations found in this study, 8 are predicted to result in the synthesis of a truncated protein caused by either a premature stop codon or a frameshift leading to a premature downstream stop codon. Remarkably, a CC → TT double transition at nucleotide 2295 was observed in three tumors from three different patients. This mutation introduces a premature stop codon at position 770 of the amino acid sequence in the second extracellular loop, immediately downstream of the transmembrane domain of the HPTC protein (Fig. 2). There have been two other nonsense mutations identified (codons 184 and 926) that give rise to stop codons resulting in truncations situated in the large extracellular loops of the protein. In one non-XP sporadic BCC that was analyzed in a parallel study, we also detected the tandem substitution GG2766AA, which results in a premature stop at codon 926 (data not shown). The two frameshifts that we detected arose from: (i) an 11-bp deletion (nucleotides 2122–2132) in exon 13 that leads to a predicted premature stop codon located 63 bp further on; (ii) a 1-bp deletion (nucleotide 3237) in exon 18 with a predicted premature stop codon located 7 bp further in the 10th transmembrane protein domain (Fig. 2).
Figure 2.
Schematic representation of the location of mutations on the HPTC protein. The HPTC protein with the predicted 12 transmembrane domains is presented. The numbers refer to mutated codons with corresponding exons in parentheses. ▴, Missense mutation. ●, Stop mutation. ♦, Frameshift mutation.
The remaining 11 alterations that we have characterized are missense mutations, and their locations on the HPTC protein are also shown Fig. 2. Interestingly, in two BCCs from unrelated patients, we have observed another tandem CC → TT transition located at codon 294, resulting in an arginine-to-cysteine change in the first extracellular loop of the protein. In summary, 17 of the 19 (89%) alterations that we have identified are base substitutions: 11 tandem CC → TT mutations, 3 C → T transitions, 1 CC → AT double base substitution, and 2 transversions (T → A and A → C). It is worth noting that all the single-base substitutions occur at bipyrimidine sequences, particularly at CC sequences that are targets for UV-induced DNA photolesions, and the majority are caused by unrepaired lesions in the nontranscribed strand (Tables 1 and 2).
Table 2.
Frequencies and distribution of hptc mutations in XP BCCs
| Mutation information | No. (%) |
|---|---|
| Total alterations | 19* |
| Deletion | 2 (11%) |
| Type of base substitutions* | |
| GC → AT | 3 (17%) |
| AT → CG | 1 (6%) |
| AT → TA | 1 (6%) |
| CC → TT | 11 (65%) |
| CC → AT | 1 (6%) |
| Total | 17 |
| Substitution location | |
| Py–Py site | 17 (100%) |
| Non-Py–Py site | 0 |
| Py–Py site location | |
| Transcribed strand | 2 (12%) |
| Nontranscribed strand | 15 (88%) |
Tandem mutations were taken as one event.
There were seven possible polymorphisms of the hptc gene characterized on the basis of the same PCR-SSCP shift obtained with DNA from tumors and healthy unexposed skin (Table 3). The polymorphisms we observed in exon 5, intron 5, and intron 14 have been reported (9, 19). The other polymorphisms that we detected in our study had not been described. These include a C → T substitution at nucleotide 1540 (exon 11) and an A → G substitution at nucleotide 2187 (exon 13), neither of which introduces modifications in the amino acid sequence. However, the remaining two polymorphisms (found in tumors and healthy skin from patients with XP), a C → T substitution at nucleotide 2161 (exon 13) and an A → G at nucleotide 3313 (exon 19) both result in amino acid changes: a proline-to-serine and a serine-to-glutamine, respectively. We also found the C2161T (exon 13) substitution in one control DNA sample from a fibroblast culture of a skin biopsy taken from a normal white individual analyzed for hptc gene polymorphisms.
Table 3.
Polymorphisms of the hptc gene
| Exon/intron | Polymorphism | Effect on coding |
|---|---|---|
| Exon 5 | A or G at 723 | No change Thr-245 |
| Intron 5 | T or C 55 bp 5′ of exon 6 | — |
| Exon 11 | C or T at 1540 | No change Leu-518 |
| Exon 13 | C or T at 2161 | Pro/Ser at 725 |
| Exon 13 | A or G at 2187 | No change Ser-733 |
| Intron 14 | G or C 8 bp 3′ of exon 14 | — |
| Exon 19 | A or G at 3313 | Ser/Glu at 1,109 |
DISCUSSION
Tumor development is a complex process that occurs either spontaneously or after exposure to genotoxic stress (20). In skin carcinogenesis, the epidermis is the primary target for the genotoxic agent, UVB radiation emitted by the sun. Molecular epidemiology studies have shown clearly that UV radiation (290–320 nm) can induce mutations, leading to the activation of oncogenes, such as the ras genes (13), or to the loss of function of the tumor suppressor gene p53 (14–16). However, the recent discovery of hptc, a gene that plays a central role both in the HH signaling pathway during embryonic development (4, 21) and that also behaves as a tumor suppressor gene (4, 9), has shed new light on tumor biology. Germ-line mutations of the hptc gene are, in fact, responsible for the NBCC syndrome. Moreover, in sporadic skin cancers, mutations in the hptc gene have been detected only in BCCs, but not in SCC (22, 23), a specificity raising many intriguing questions.
Our data suggest that a number of hptc gene polymorphisms may exist (Table 3); among them, a C → T at nucleotide 2161 was reported to be a mutation by Wolter et al. (19). However, our data show the presence of the same mutation in DNA from two tumors and two unexposed healthy skin samples taken from 34 XP individuals and in unexposed skin from one normal white individual. We are thus confident that this C → T alteration is a real polymorphism of the hptc gene. The mutation seen in exon 19 results in a serine-to-glutamine change at amino acid 1109. A glutamine at the same position is also found in mouse, chicken, and Drosophila, indicating that it is an evolutionarily important hptc gene polymorphism.
Analysis of the hptc gene in BCCs from patients with XP has identified 19 modifications in 16 of 22 (73%) BCCs. Of these alterations, 89% (17 of 19) are point mutations, and only 11% (2 of 19) are deletions (Tables 1 and 2). These results differ from those observed in sporadic BCCs in which 68% of alterations were point mutations and 32% were rearrangements (9). In NBCC, the majority (64–71%) of germ-line hptc mutations are rearrangements (18, 24). These differences may be explained by the absence of DNA repair in the cells of patients with XP, resulting in the higher level of UV-induced point mutations, which is the type of mutation that we find to be predominant in our study. Among the 17 point mutations we found, 11 were missense point mutations all occurring at amino acid residues conserved during evolution. These residues may play key roles in HPTC protein function, in binding sonic HH (SHH) at the extracellular loops (6, 7), in membrane transport for residues located in the predicted transmembrane domains, or in signal transduction, as was suggested (24) for residues of the C-terminal intracellular region of the HPTC protein (Fig. 2).
Very interestingly, some missense mutations were detected twice (e.g., the CC867TT in exon 6). Although the number of samples in our study is small, given the length of the hptc gene, it is unlikely that recurrent mutations occur at random. It can be supposed then that, depending on the sequence context, some nucleotides have a higher mutagenic potential. The induced amino acid change could inactivate HPTC protein function, and mutated cells could then acquire growth advantages (8). Further studies of cell lines transformed with different mutant HPTC proteins should provide insight on the role of the different protein regions. In fact, the high levels of point mutations found in the hptc gene of XP skin tumors, in contrast to those seen in sporadic BCCs or in NBCC syndrome (18, 24), can be extremely useful in defining the codons of the protein whose modification allows cell transformation. Indeed, hptc gene alterations in XP even could be considered as site-directed mutagenesis in vivo, allowing one to pinpoint the important functional domains of the protein.
Some hptc alterations leading to the introduction of stop codons also have a degree of recurrence, which may be caused by the sequence context. The CC2295TT tandem mutation affecting codon 770 was found in three BCCs analyzed and may correspond to a mutation hotspot of the hptc gene, because, with our small number of XP samples, the probability of finding 3 independent mutations at the same site is lower than 4 × 10−3. This number would be even lower if one considered all the hptc mutations detected up to now. The GG2766AA tandem mutation at codon 926 we found in an XP BCC also was detected in a sporadic non-XP BCC (data not shown). Mutations at such possible hotspots would result in the synthesis of a truncated and nonfunctional protein.
In our study, 79% (15 of 19) of the mutations detected were UV-specific (11 CC → TT tandem mutations and 4 C → T transitions, including the one found in the CC → AT double mutation), and all of these 15 were located at bipyrimidine sites (Tables 1 and 2). These mutations occur in the absence of efficient repair of UV-induced DNA lesions, which are mostly cyclobutane pyrimidine dimers and 6-4 pyrimidine pyrimidones. Our results, obtained in BCCs from patients with XP, thus clearly show that unrepaired photolesions resulting in the UV-induced mutations on the hptc gene play a decisive role in tumor formation. In the study analyzing sporadic BCCs (9), the level of UV-induced mutations was significantly lower (44% vs. 79%) than that seen in XP BCCs (P = 0.04 in the Fisher two-sided test). Interestingly, 15 of 17 of the base-pair substitutions we found in our XP tumors are located in the nontranscribed strand of the hptc gene, indicating that there is preferential repair of the transcribed strand in these patients with XP. This conclusion contrasts with the location of mutations seen in tumors from the non-XP population, in which mutations are distributed equally on both strands of the DNA. In fact, our tumor samples are mostly from patients with XP belonging to the complementation group C, known to be efficient in the repair of only the transcribed strand of the DNA.
Together with our previous studies of the status of p53 in XP epitheliomas (14, 15), the present work strengthens the link between UV-induced DNA lesions, DNA-repair capacity, and tumorigenesis. Moreover, 14 tumor DNAs analyzed here for hptc modifications already have been screened in our laboratory for p53 mutations (14, 15), and 4 tumors contained only the hptc gene mutation, 1 had only a mutated p53 gene, and 2 did not have either hptc or p53 mutations. In contrast, 7 of 14 tumor DNAs exhibited mutations in both the hptc and p53 genes. Interestingly, all the p53 mutations were UV-specific, which not only further indicates the causal role of UV-specific lesions in skin-cancer development but also lends additional evidence of the multiplicity of genetic alterations leading to tumor formation. Which gene alteration, p53 or hptc, if either, primarily triggers the onset of BCC development remains to be determined. Different authors propose that hptc could be a gatekeeper gene for BCCs (8, 25, 26), suggesting that alterations of hptc occur at an early stage of the carcinogenic process. This hypothesis is supported by several pieces of evidence. First, germ-line mutations of hptc are associated with a high predisposition to develop BCCs at an early age in patients with NBCC. Second, hptc mutations have been detected in trichoepithelioma, a benign cutaneous tumor that can be associated with BCC. Third, it has been shown in animal models that disruption of the HH signaling pathway is sufficient to induce BCCs within the first few days of the skin development in the embryo (25).
At present, there is no direct evidence to affirm that mutations of hptc arise earlier than mutations of p53 in BCCs. It is interesting to note that, in a recent study (27), it was found that p53 alterations are an early event in progression toward SCC in the skin, whereas malignant development involves loss of heterozygosity and alterations of genes located in the long arm of chromosome 9. In fact, the 9q22.3 region contains the hptc gene, which has never been found to be modified in SCC (data not shown and refs. 22 and 23), even though p53 mutations are detected in both BCCs and SCCs. This fact raises the intriguing question of whether the expression of hptc could be specific to a keratinocyte subpopulation giving rise specifically to BCCs. Loss-of-heterozygosity analysis has shown that inactivation of both hptc alleles does occur in sporadic BCC development (9), and it will be important to confirm this inactivation in XP BCCs.
Our data show a high level of mutations (73%) of the hptc gene in XP skin cancers and also strongly indicate the key role played by hptc in BCC formation. However, several recent studies also have explained the role of other members of the HH pathway in BCC development. Overexpression of shh in the epidermis of transgenic mice results in BCC formation (25), although mice do not naturally develop BCCs. Furthermore, human epidermal keratinocytes retrovirally transduced by a shh-expressing vector can develop into BCCs after grafting onto the nude mouse (28). The role of the HH pathway also is illustrated well by induction of BCC in embryonic frog epidermis after transgenic overexpression of Gli1 (29), a downstream effector of the HH pathway. The analysis of the DNAs of other members of the HH signaling pathway should help to gain additional insight into the specific roles they may play in the development of BCC and/or SCC. For instance, it has been shown recently that smo, the gene encoding the transmembrane activator of the shh pathway and whose expression is constitutively repressed by hptc in the absence of shh, may be mutated in BCC (30). Likewise, mutations of shh also were found in sporadic BCCs and other tumors (25).
Our analysis of epidermal tumors from patients with XP has substantiated further the role of unrepaired UV-induced DNA lesions in skin carcinogenesis. The key role played by hptc in BCC development is marked by the high rate of mutations found in our series of XP BCCs. Taken together, our results incite us to examine further how unrepaired UV lesions affect other components of the HH signaling pathway. Indeed, analysis of skin tumors from the cancer-prone patients with XP should help to define more easily which members of the signaling pathway are involved directly in the skin carcinogenesis progression in general.
Acknowledgments
We are grateful to all clinicians who sent us tumor samples. This work was supported by grants from the Association pour la Recherche sur le Cancer (Villejuif, France). N.B. was supported by the Assistance Publique-Hôpitaux de Paris, and S.Q. was supported by the Ligue Nationale Contre Le Cancer.
ABBREVIATIONS
- XP
xeroderma pigmentosum
- BCC
basal-cell carcinoma
- NBCC
nevoid BCC
- SSCP
single-strand conformation polymorphism
- SCC
squamous-cell carcinoma
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Miller S. J Am Acad Dermatol. 1991;24:1–13. doi: 10.1016/0190-9622(91)70001-i. [DOI] [PubMed] [Google Scholar]
- 2.Gorlin R. Medicine (Baltimore) 1987;66:98–113. doi: 10.1097/00005792-198703000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Goeteyn M, Geerts M L, Kint A, De Weert J. Arch Dermatol. 1994;130:337–342. [PubMed] [Google Scholar]
- 4.Hahn H, Wicking C, Zaphiropoulous P, Gailani M, Shanley S, Chidambaram A, Vorechovsky I, Holmberg E, Unden A, Gillies S, et al. Cell. 1996;85:841–851. doi: 10.1016/s0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- 5.Johnson R, Rothman A, Xie J, Goodrich L, Bare J, Bonifas J, Quinn A, Myers R, Cox D, Epstein E J, et al. Science. 1996;272:1668–1671. doi: 10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- 6.Marigo V, Davey R, Zuo Y, Cunningham J, Tabin C. Nature (London) 1996;384:176–179. doi: 10.1038/384176a0. [DOI] [PubMed] [Google Scholar]
- 7.Stone D, Hynes M, Armanini M, Swanson T, Gu Q, Johnson R, Scott M, Pennica D, Goddard A, Phillips H, et al. Nature (London) 1996;384:129–134. doi: 10.1038/384129a0. [DOI] [PubMed] [Google Scholar]
- 8.Dean M. Biochim Biophys Acta. 1997;1332:M42–M52. doi: 10.1016/s0304-419x(96)00043-1. [DOI] [PubMed] [Google Scholar]
- 9.Gailani M, Stahle-Backdahl M, Leffell D, Glynn M, Zaphiropoulos P, Pressman C, Unden A, Dean M, Brash D, Bale A, et al. Nat Genet. 1996;14:78–81. doi: 10.1038/ng0996-78. [DOI] [PubMed] [Google Scholar]
- 10.Stary A, Sarasin A. Cancer Surv. 1996;26:155–171. [PubMed] [Google Scholar]
- 11.Drobetsky E A, Grosovsly A J, Glickman B W. Proc Natl Acad Sci USA. 1987;84:9103–9107. doi: 10.1073/pnas.84.24.9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutchinson F. Mutat Res. 1994;309:11–15. doi: 10.1016/0027-5107(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 13.Daya-Grosjean L, Robert C, Drougard C, Suarez H, Sarasin A. Cancer Res. 1993;53:1625–1629. [PubMed] [Google Scholar]
- 14.Giglia G, Dumaz N, Drougard C, Avril M, Daya-Grosjean L, Sarasin A. Cancer Res. 1998;58:4402–4409. [PubMed] [Google Scholar]
- 15.Dumaz N, Drougard C, Sarasin A, Daya-Grosjean L. Proc Natl Acad Sci USA. 1993;90:10529–10533. doi: 10.1073/pnas.90.22.10529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ziegler A, Sarason A S, Lefell D J, Simon J A, Sharna H W, Kimmelman J, Pemington L, Jacks T, Brash D E. Nature (London) 1994;372:773–776. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]
- 17.Daya-Grosjean L, Dumaz N, Sarasin A. J Photochem Photobiol B. 1995;28:115–124. doi: 10.1016/1011-1344(95)07130-t. [DOI] [PubMed] [Google Scholar]
- 18.Chidambaram A, Goldstein A, Gailani M, Gerrard B, Bale S, DiGiovanna J, Bale A, Dean M. Cancer Res. 1996;56:4599–4601. [PubMed] [Google Scholar]
- 19.Wolter M, Reifenberger J, Sommer C, Ruzicka T, Reifenberger G. Cancer Res. 1997;57:2581–2585. [PubMed] [Google Scholar]
- 20.Perera F P. J Natl Cancer Inst. 1996;88:496–509. doi: 10.1093/jnci/88.8.496. [DOI] [PubMed] [Google Scholar]
- 21.Goodrich L, Johnson R, Milenkovic L, McMahon J, Scott M. Genes Dev. 1996;10:301–312. doi: 10.1101/gad.10.3.301. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki K, Daigo Y, Fukudi S, Tokino J, Isomura M, Isono K, Wainwright B, Nakamura Y. Jpn J Cancer Res (GANN) 1997;88:225–228. doi: 10.1111/j.1349-7006.1997.tb00370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eklund L K, Lindström E, Unden A B, Lundh-Rozell B, Stahle-Backdahl M, Zaphiropoulos P G, Toftgard R, Soderkvist P. Mol Carcinog. 1998;21:87–92. doi: 10.1002/(sici)1098-2744(199802)21:2<87::aid-mc2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 24.Wicking C, Shanley S, Smyth I, Gillies S, Negus K, Graham S, Suthers G, Haites N, Edwards M, Wainwright B, et al. Am J Hum Genet. 1997;60:21–26. [PMC free article] [PubMed] [Google Scholar]
- 25.Oro A, Higgins K, Hu Z, Bonifas J, Epstein E, Scott M. Science. 1997;276:817–821. doi: 10.1126/science.276.5313.817. [DOI] [PubMed] [Google Scholar]
- 26.Sidransky D. Nat Genet. 1996;14:7–8. doi: 10.1038/ng0996-7. [DOI] [PubMed] [Google Scholar]
- 27.Ahmadian A, Ren Z-P, Williams C, Pontén F, Odeberg J, Pontén J, Uhlén M, Lundeberg J. Oncogene. 1998;17:1837–1843. doi: 10.1038/sj.onc.1202080. [DOI] [PubMed] [Google Scholar]
- 28.Fan H, Oro A E, Scott M P, Khavari P A. Nat Med. 1997;3:788–792. doi: 10.1038/nm0797-788. [DOI] [PubMed] [Google Scholar]
- 29.Dahmane N, Lee J, Robins P, Heller P, Ruizi Altaba A. Nature (London) 1997;389:876–881. doi: 10.1038/39918. [DOI] [PubMed] [Google Scholar]
- 30.Xie J, Murone M, Luoh S-M, Ryan A, Gu Q, Zhang C, Bonifas J M, Lam C-W, Hynes M, Goddard A, et al. Nature (London) 1998;391:90–92. doi: 10.1038/34201. [DOI] [PubMed] [Google Scholar]