Abstract
Flagellar hook proteins from Salmonella and Escherichia coli were dissociated in acid and purified by diethylamino-ethyl-cellulose column chromatography. These two proteins had the same electrophoretic mobility in sodium dodecyl sulfate-polyacrylamide gels. However, analytical electrofocusing patterns showed that these proteins had different isoelectric points (4.7 for Salmonella typhimurium and 4.4 for E. coli). Immunodiffusion and immuno-electron microscopy carried out with antisera prepared against purified hook proteins from S. typhimurium and E. coli showed that these antisera reacted with both hooks. Affinity chromatography allowed separation of antibodies specific for hook proteins from each bacterial species. These results indicate that the hook proteins share common antigenic determinants as well as specific antigens, although the specificity is not quantitatively resolved. From comparisons of the amino acid composition of the hook proteins and flagellins, it was concluded that the differences between flagellins from S. typhimurium and E. coli were larger than those between hook proteins from these species.
Full text
PDF
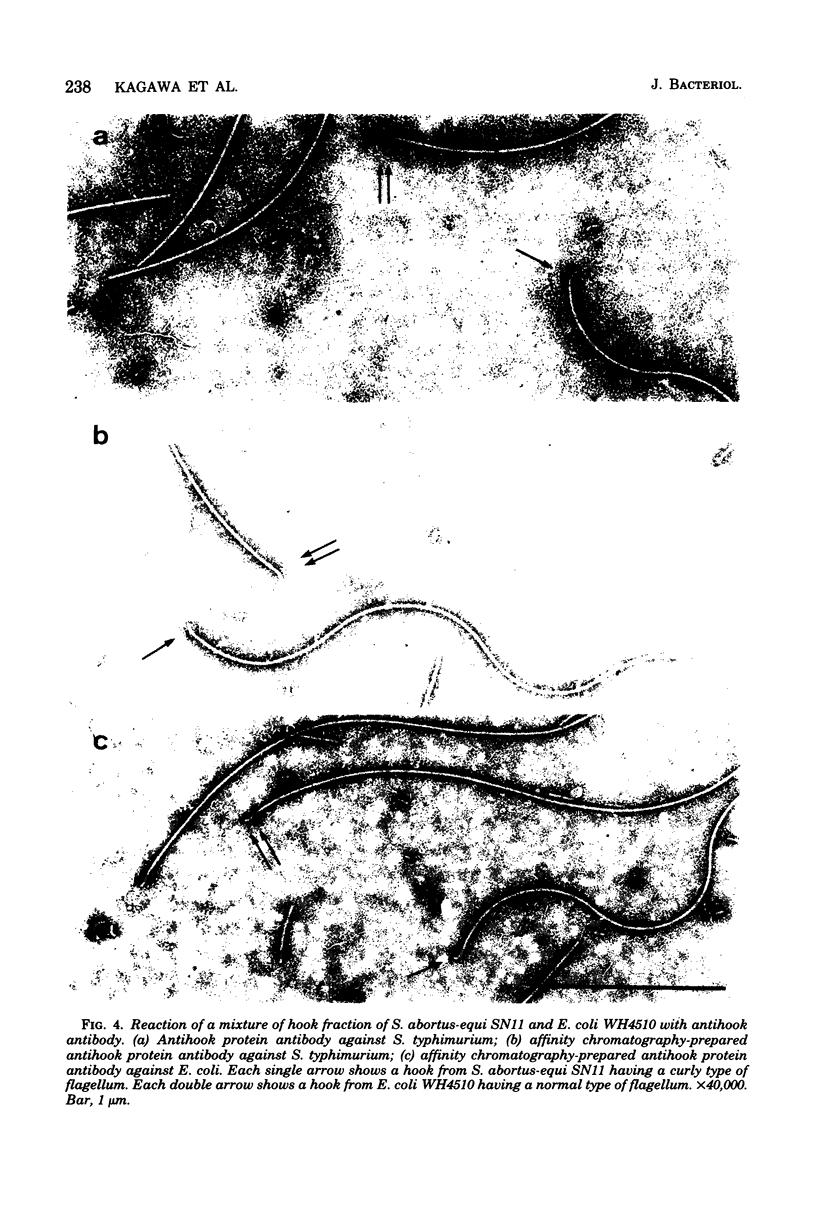


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abram D., Mitchen J. R., Koffler H., Vatter A. E. Differentiation within the bacterial flagellum and isolation of the proximal hook. J Bacteriol. 1970 Jan;101(1):250–261. doi: 10.1128/jb.101.1.250-261.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delange R. J., Chang J. Y., Shaper J. H., Martinez R. J., Komatsu S. K., Glazer A. N. On the amino-acid sequence of flagellin from Bacillus subtilis 168: comparison with other bacterial flagellins. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3428–3431. doi: 10.1073/pnas.70.12.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmitt K., Simon M. Antigenic nature of bacterial flagellar hook structures. Infect Immun. 1970 Feb;1(2):212–213. doi: 10.1128/iai.1.2.212-213.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Hori H. Evolution of 5sRNA. J Mol Evol. 1975 Dec 31;7(1):75–86. doi: 10.1007/BF01732181. [DOI] [PubMed] [Google Scholar]
- Hotani H., Ooi T., Kagawa H., Asakura S., Yamaguchi S. Biochemical evidence for identical primary structure of P-filament and flagellin. Biochim Biophys Acta. 1970 Jul 27;214(1):207–215. doi: 10.1016/0005-2795(70)90085-1. [DOI] [PubMed] [Google Scholar]
- Iino T. Genetics and chemistry of bacterial flagella. Bacteriol Rev. 1969 Dec;33(4):454–475. doi: 10.1128/br.33.4.454-475.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joys T. M., Rankis V. The primary structure of the phase-1 flagellar protein of Salmonella typhimurium. I. The tryptic peptides. J Biol Chem. 1972 Aug 25;247(16):5180–5193. [PubMed] [Google Scholar]
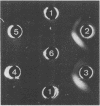
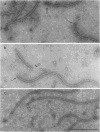
- Kagawa H., Asakura S., Iino T. Serological study of bacterial flagellar hooks. J Bacteriol. 1973 Mar;113(3):1474–1481. doi: 10.1128/jb.113.3.1474-1481.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa H., Owaribe K., Asakura S., Takahashi N. Flagellar hook protein from Salmonella SJ25. J Bacteriol. 1976 Jan;125(1):68–73. doi: 10.1128/jb.125.1.68-73.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komeda Y., Silverman M., Simon M. Identification of the structural gene for the hook subunit protein of Escherichia coli flagella. J Bacteriol. 1978 Jan;133(1):364–371. doi: 10.1128/jb.133.1.364-371.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn A. M. Simple immunological labelling method for electron microscopy and its application to the study of filamentous appendages of bacteria. Nature. 1967 Jun 10;214(5093):1151–1152. doi: 10.1038/2141151a0. [DOI] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Metzger H., Shapiro M. B., Mosimann J. E., Vinton J. E. Assessment of compositional relatedness between proteins. Nature. 1968 Sep 14;219(5159):1166–1168. doi: 10.1038/2191166a0. [DOI] [PubMed] [Google Scholar]
- Patterson-Delafield J., Martinez R. J., Stocker B. A., Yamaguchi S. A new fla gene in Salmonella typhimurium--flaR--and its mutant phenotype-superhooks. Arch Mikrobiol. 1973 Mar 26;90(2):107–120. doi: 10.1007/BF00414513. [DOI] [PubMed] [Google Scholar]
- Silverman M. R., Simon M. I. Flagellar assembly mutants in Escherichia coli. J Bacteriol. 1972 Nov;112(2):986–993. doi: 10.1128/jb.112.2.986-993.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Iino T., Horiguchi T., Yamaguchi S. Incomplete flagellar structures in nonflagellate mutants of Salmonella typhimurium. J Bacteriol. 1978 Feb;133(2):904–915. doi: 10.1128/jb.133.2.904-915.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S., Iino T. Serological and finger-printing analyses of mutant flagellar antigens of Salmonella. J Gen Microbiol. 1970 Dec;64(3):311–318. doi: 10.1099/00221287-64-3-311. [DOI] [PubMed] [Google Scholar]