Abstract
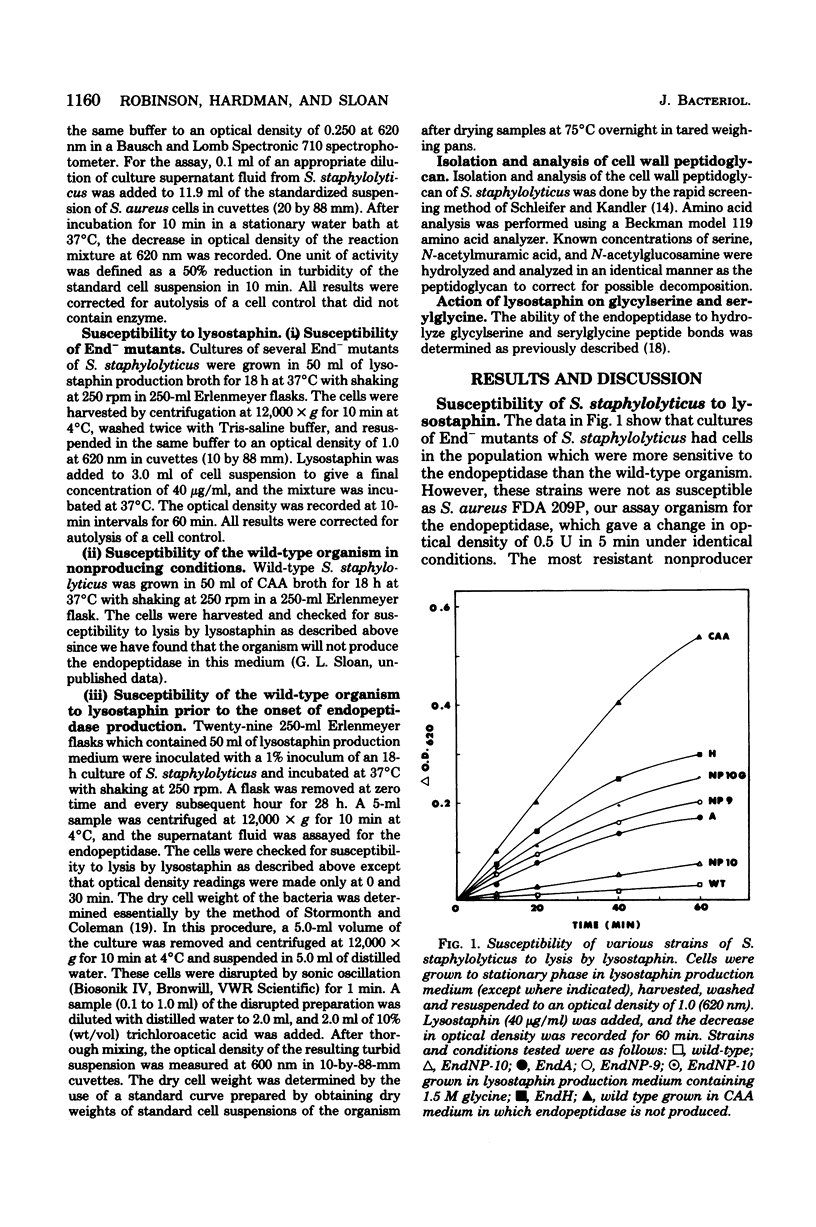
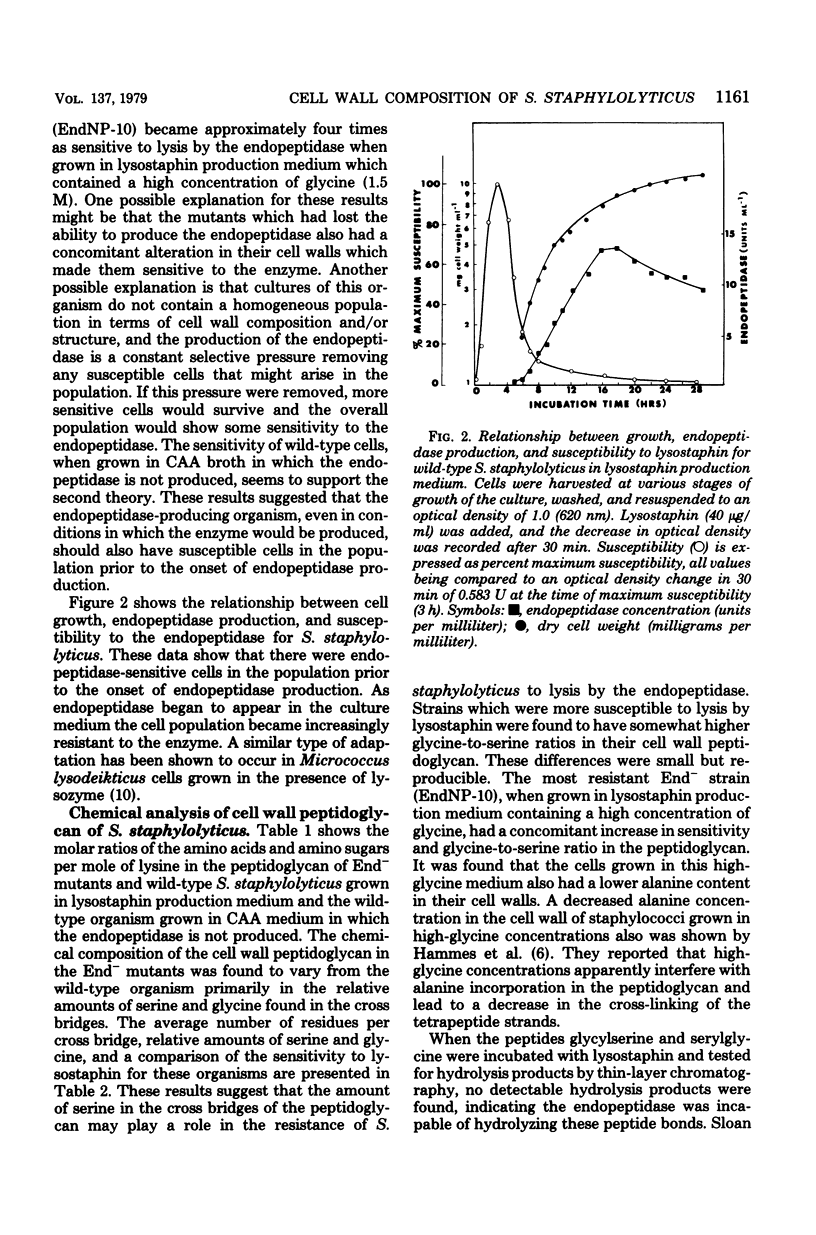
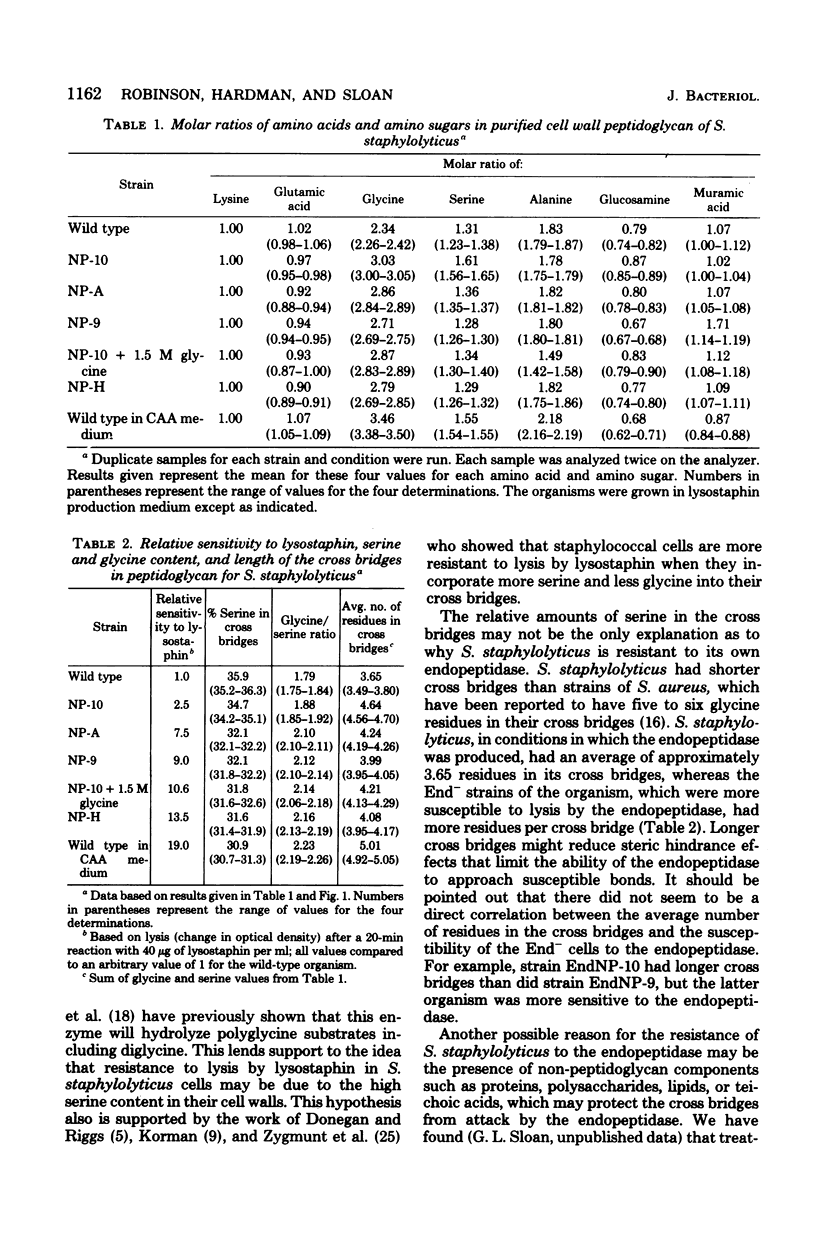
Mutants of Staphylococcus staphylolyticus incapable of producing an extracellular staphylolytic glycylglycine endopeptidase were isolated and found to have cells in the population susceptible to lysis by this enzyme, as did the wild-type organism under conditions in which the endopeptidase was not produced. These results suggest that cultures of this organism normally contain a heterogeneous population of cells with regard to cell wall composition and susceptibility to the enzyme. Production of the endopeptidase appears to act as a selective pressure which removes the susceptible cells in the population as the enzyme appears in the medium. A comparison of the peptidoglycan of the wild-type organism grown under conditions in which the endopeptidase was produced with that of this organism grown under nonproducing conditions and with those of endopeptidase-less mutants showed that in the presence of the endopeptidase the cell population had peptidoglycan with shorter peptide cross bridges and a greater percentage of serine in these cross bridges than was found in cells grown in the absence of the enzyme. The inability of the endopeptidase to hydrolyze glycylserine and serylglycine peptide bonds suggests that at least part of the resistance this organism has to the endopeptidase is due to relative amounts of serine found in the peptide cross bridges of some cells in the population.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWDER H. P., ZYGMUNT W. A., YOUNG J. R., TAVORMINA P. A. LYSOSTAPHIN: ENZYMATIC MODE OF ACTION. Biochem Biophys Res Commun. 1965 Apr 23;19:383–389. doi: 10.1016/0006-291x(65)90473-0. [DOI] [PubMed] [Google Scholar]
- Browder H. P., Tavormina P. A., Zygmunt W. A. Optical configuration of staphylococcal cell wall serine. J Bacteriol. 1968 Oct;96(4):1452–1453. doi: 10.1128/jb.96.4.1452-1453.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROPP C. B., HARRISON E. F. THE IN VITRO EFFECT OF LYSOSTAPHIN ON CLINICAL ISOLATES OF STAPHYLOCOCCUS AUREUS. Can J Microbiol. 1964 Dec;10:823–828. doi: 10.1139/m64-107. [DOI] [PubMed] [Google Scholar]
- Donegan E. A., Riggs H. G., Jr In vitro incorporation of serine into the staphylococcal cell wall. Infect Immun. 1974 Jul;10(1):264–269. doi: 10.1128/iai.10.1.264-269.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes W., Schleifer K. H., Kandler O. Mode of action of glycine on the biosynthesis of peptidoglycan. J Bacteriol. 1973 Nov;116(2):1029–1053. doi: 10.1128/jb.116.2.1029-1053.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen O. J., Grov A. Studies on lysostaphin. Separation and characterization of three enzymes. Eur J Biochem. 1973 Oct 5;38(2):293–300. doi: 10.1111/j.1432-1033.1973.tb03061.x. [DOI] [PubMed] [Google Scholar]
- KLOOS W. E., PATTEE P. A. A BIOCHEMICAL CHARACTERIZATION OF HISTIDINE-DEPENDENT MUTANTS OF STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1965 May;39:185–194. doi: 10.1099/00221287-39-2-185. [DOI] [PubMed] [Google Scholar]
- Korman R. Z. Elevated cell wall serine in pleiotropic staphylococcal mutants. J Bacteriol. 1966 Sep;92(3):762–768. doi: 10.1128/jb.92.3.762-768.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITWACK G. Development of Micrococcus lysodeikticus resistant to lysozyme. Nature. 1958 May 10;181(4619):1348–1350. doi: 10.1038/1811348c0. [DOI] [PubMed] [Google Scholar]
- SCHINDLER C. A., SCHUHARDT V. T. LYSOSTAPHIN: A NEW BACTERIOLYTIC AGENT FOR THE STAPHYLOCOCCUS. Proc Natl Acad Sci U S A. 1964 Mar;51:414–421. doi: 10.1073/pnas.51.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHINDLER C. A., SCHUHARDT V. T. PURIFICATION AND PROPERTIES OF LYSOSTAPHIN--A LYTIC AGENT FOR STAPHYLOCOCCUS AUREUS. Biochim Biophys Acta. 1965 Feb 15;97:242–250. doi: 10.1016/0304-4165(65)90088-7. [DOI] [PubMed] [Google Scholar]
- Schindler C. A. Staphylococcal strains with relation to lysostaphin sensistivity. Nature. 1966 Mar 26;209(5030):1368–1369. doi: 10.1038/2091368a0. [DOI] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer K. H., Kocur M. Classification of staphylococci based on chemical and biochemical properties. Arch Mikrobiol. 1973 Oct 4;93(1):65–85. doi: 10.1007/BF00666081. [DOI] [PubMed] [Google Scholar]
- Schleifer K. H., Schumacher-Perdreau F., Götz F., Popp B. Chemical and biochemical studies for the differentiation of coagulase-positive staphylococci. Arch Microbiol. 1976 Nov 2;110(23):263–270. doi: 10.1007/BF00690237. [DOI] [PubMed] [Google Scholar]
- Sloan G. L., Smith E. C., Lancaster J. H. Lysostaphin endopeptidase-catalysed transpeptidation reactions of the imino-transfer type. Biochem J. 1977 Oct 1;167(1):293–296. doi: 10.1042/bj1670293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strominger J. L., Ghuysen J. M. Mechanisms of enzymatic bacteriaolysis. Cell walls of bacteri are solubilized by action of either specific carbohydrases or specific peptidases. Science. 1967 Apr 14;156(3772):213–221. doi: 10.1126/science.156.3772.213. [DOI] [PubMed] [Google Scholar]
- Tipper D. J., Berman M. F. Structures of the cell wall peptidoglycans of Staphylococcus epidermidis Texas 26 and Staphylococcus aureus Copenhagen. I. Chain length and average sequence of cross-bridge peptides. Biochemistry. 1969 May;8(5):2183–2192. doi: 10.1021/bi00833a060. [DOI] [PubMed] [Google Scholar]
- Wadstrom T., Vesterberg O. Studies on endo-beta-acetylglucosaminidase, staphylolytic peptidase, and N-acetylmuramyl-L-alanine amidase in lysostaphin and from Staphylococcus aureus. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(2):248–264. doi: 10.1111/j.1699-0463.1971.tb02152.x. [DOI] [PubMed] [Google Scholar]
- Zygmunt W. A., Browder H. P., Tavormina P. A. Susceptibility of coagulase-negative staphylococci to lysostaphin and other antibiotics. Appl Microbiol. 1968 Aug;16(8):1168–1173. doi: 10.1128/am.16.8.1168-1173.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]



