Abstract
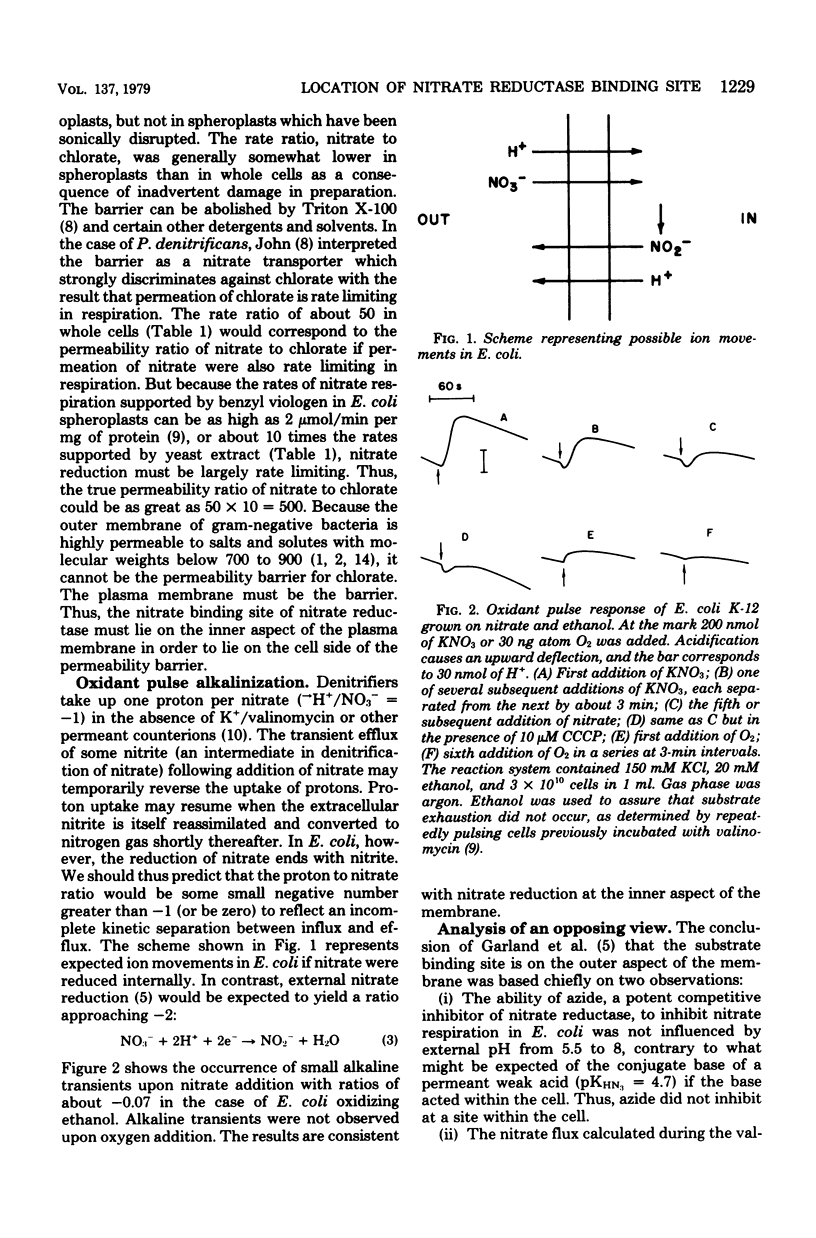
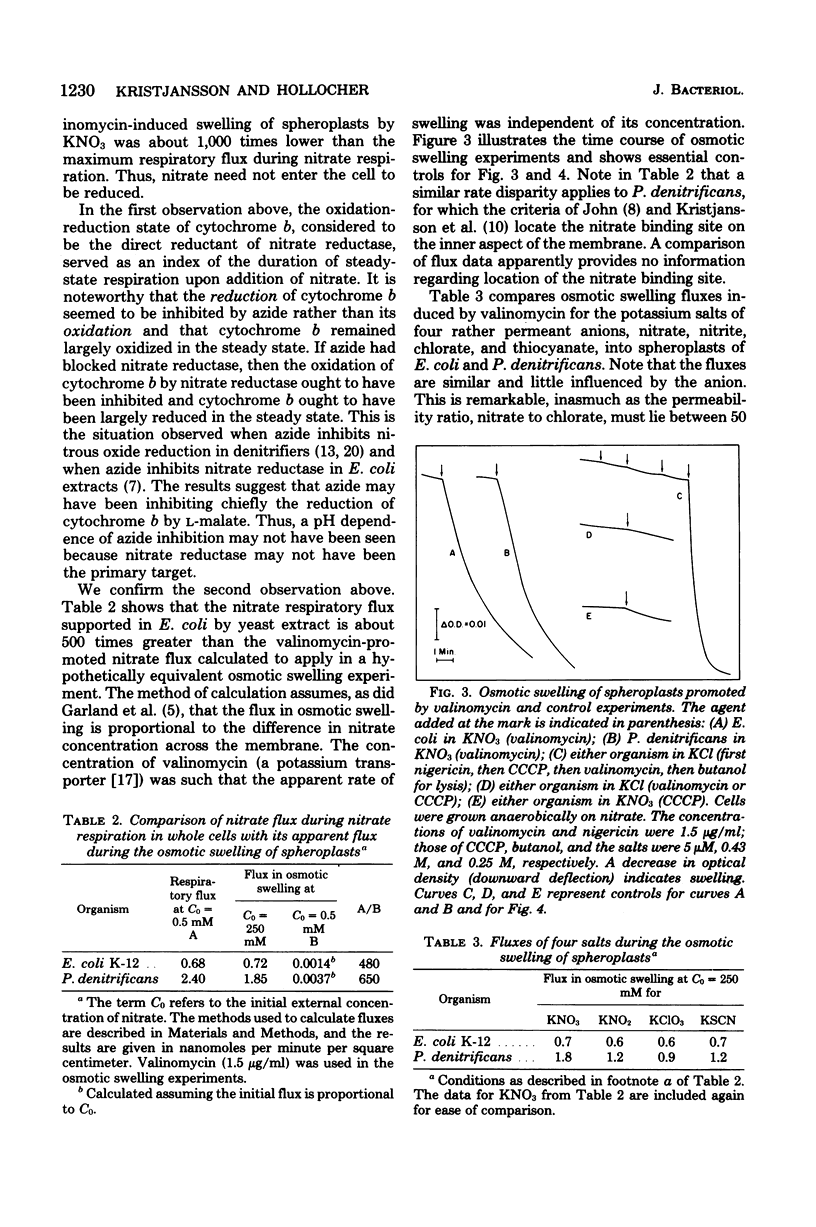
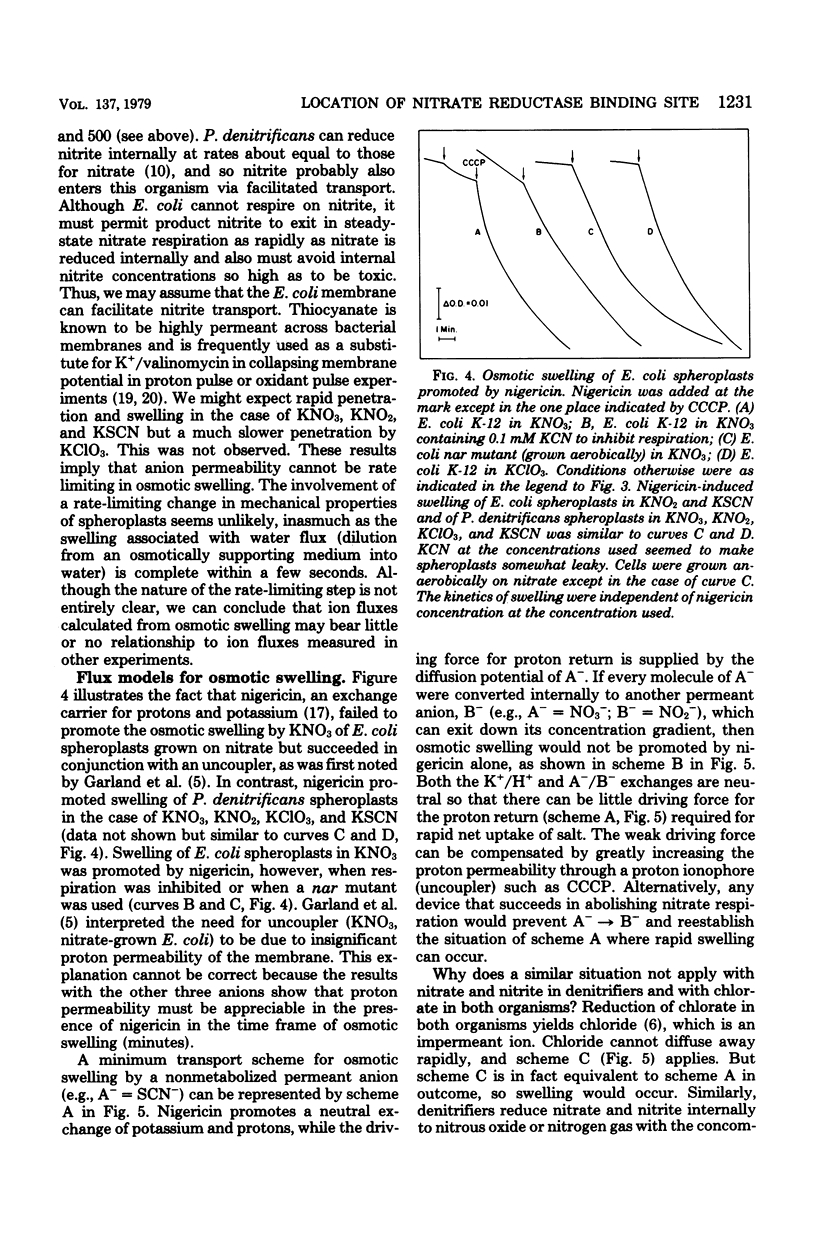
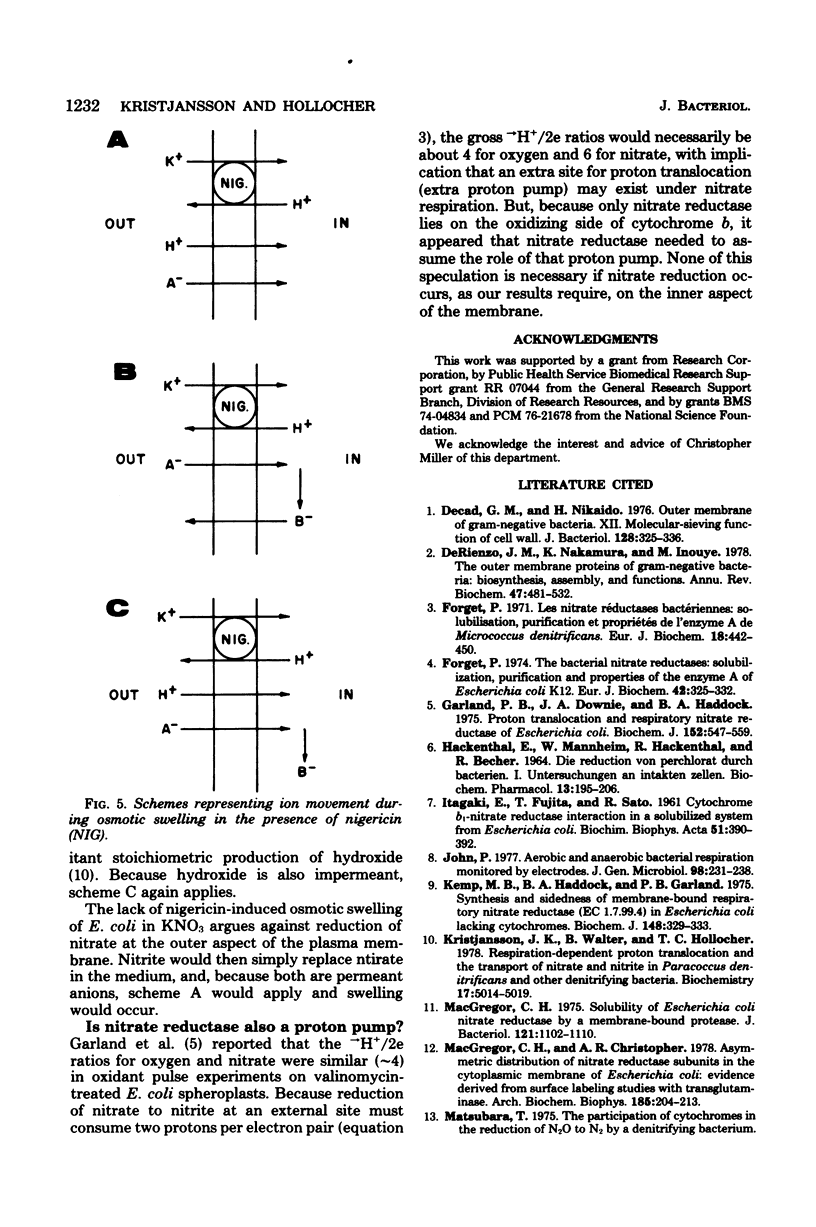
Escherichia coli grown anaerobically on nitrate exhibited the same transport barrier to reduction of chlorate, relative to nitrate, as that exhibited by Paracoccus denitrificans. This establishes that the nitrate binding site of nitrate reductase (EC 1.7.99.4) in E. coli must also lie on the cell side of the nitrate transporter which is associated with the plasma membrane. Because nitrate reductase is membrane bound, the nitrate binding site is thus located on the inner aspect of the membrane. Nitrate pulse studies on E. coli in the absence of valinomycin showed a small transient alkalinization (leads to H+/NO3- congruent to --0.07) which did not occur with oxygen pulses. By analogy with P. denitrificans, the alkaline transient is interpreted to arise from proton-linked nitrate uptake which is closely followed by nitrite efflux. The result is consistent with internal reduction of nitrate, whereas external reduction would be expected to give leads to H+/NO3-ratios approaching --2.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Decad G. M., Nikaido H. Outer membrane of gram-negative bacteria. XII. Molecular-sieving function of cell wall. J Bacteriol. 1976 Oct;128(1):325–336. doi: 10.1128/jb.128.1.325-336.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRienzo J. M., Nakamura K., Inouye M. The outer membrane proteins of Gram-negative bacteria: biosynthesis, assembly, and functions. Annu Rev Biochem. 1978;47:481–532. doi: 10.1146/annurev.bi.47.070178.002405. [DOI] [PubMed] [Google Scholar]
- Forget P. Les nitrate-réductases bactériennes. Solubilisation, purification et propriétés de l'enzyme A de Micrococcus denitrificans. Eur J Biochem. 1971 Feb 1;18(3):442–450. doi: 10.1111/j.1432-1033.1971.tb01262.x. [DOI] [PubMed] [Google Scholar]
- Forget P. The bacterial nitrate reductases. Solubilization, purification and properties of the enzyme A of Escherichia coli K 12. Eur J Biochem. 1974 Mar 1;42(2):325–332. doi: 10.1111/j.1432-1033.1974.tb03343.x. [DOI] [PubMed] [Google Scholar]
- Garland P. B., Downie J. A., Haddock B. A. Proton translocation and the respiratory nitrate reductase of Escherichia coli. Biochem J. 1975 Dec;152(3):547–559. doi: 10.1042/bj1520547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HACKENTHAL E., MANNHEIM W., HACKENTHAL R., BECHER R. DIE REDUKTION VON PERCHLORAT DURCH BAKTERIEN. I. UNTERSUCHUNGEN AN INTAKTEN ZELLEN. Biochem Pharmacol. 1964 Feb;13:195–206. doi: 10.1016/0006-2952(64)90137-6. [DOI] [PubMed] [Google Scholar]
- John P. Aerobic and anaerobic bacterial respiration monitored by electrodes. J Gen Microbiol. 1977 Jan;98(1):231–238. doi: 10.1099/00221287-98-1-231. [DOI] [PubMed] [Google Scholar]
- Kristjansson J. K., Walter B., Hollocher T. C. Respiration-dependent proton translocation and the transport of nitrate and nitrite in Paracoccus denitrificans and other denitrifying bacteria. Biochemistry. 1978 Nov 14;17(23):5014–5019. doi: 10.1021/bi00616a024. [DOI] [PubMed] [Google Scholar]
- MacGregor C. H., Christopher A. R. Asymmetric distribution of nitrate reductase subunits in the cytoplasmic membrane of Escherichia coli: evidence derived from surface labeling studies with transglutaminase. Arch Biochem Biophys. 1978 Jan 15;185(1):204–213. doi: 10.1016/0003-9861(78)90160-1. [DOI] [PubMed] [Google Scholar]
- MacGregor C. H. Solubilization of Escherichia coli nitrate reductase by a membrane-bound protease. J Bacteriol. 1975 Mar;121(3):1102–1110. doi: 10.1128/jb.121.3.1102-1110.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara T. The participation of cytochromes in the reduction of N20 to N2 by a denitryfying bacterium. J Biochem. 1975 Mar;77(3):627–632. doi: 10.1093/oxfordjournals.jbchem.a130764. [DOI] [PubMed] [Google Scholar]
- Nakae T., Nikaido H. Outer membrane as a diffusion barrier in Salmonella typhimurium. Penetration of oligo- and polysaccharides into isolated outer membrane vesicles and cells with degraded peptidoglycan layer. J Biol Chem. 1975 Sep 25;250(18):7359–7365. [PubMed] [Google Scholar]
- PICHINOTY F. A PROPOS DES NITRATE-R'EDUCTASES D'UNE BACT'ERIE D'ENITRIFIANTE. Biochim Biophys Acta. 1964 Aug 26;89:378–381. [PubMed] [Google Scholar]
- Pichinoty F. Les nitrate-réductases bactériennes. I. Substrats, état particulaire et inhibiteurs de l'enzyme A. Arch Mikrobiol. 1969;68(1):51–64. [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- Scholes P., Mitchell P. Respiration-driven proton translocation in Micrococcus denitrificans. J Bioenerg. 1971 Sep;1(3):309–323. doi: 10.1007/BF01516290. [DOI] [PubMed] [Google Scholar]
- St John R. T., Hollocher T. C. Nitrogen 15 tracer studies on the pathway of denitrification in Pseudomonas aeruginosa. J Biol Chem. 1977 Jan 10;252(1):212–218. [PubMed] [Google Scholar]
- Synthesis and sideedness of membrane-bound respiratory nitrate reductase (EC1.7.99.4) in Escherichia coli lacking cytochromes. Biochem J. 1975 May;148(2):329–333. [PMC free article] [PubMed] [Google Scholar]
- Walter B., Sidransky E., Kristjansson J. K., Hollocher T. C. Inhibition of denitrification by uncouplers of oxidative phosphorylation. Biochemistry. 1978 Jul 25;17(15):3039–3045. doi: 10.1021/bi00608a015. [DOI] [PubMed] [Google Scholar]


