Abstract
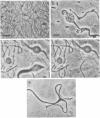
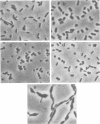
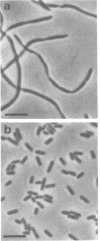
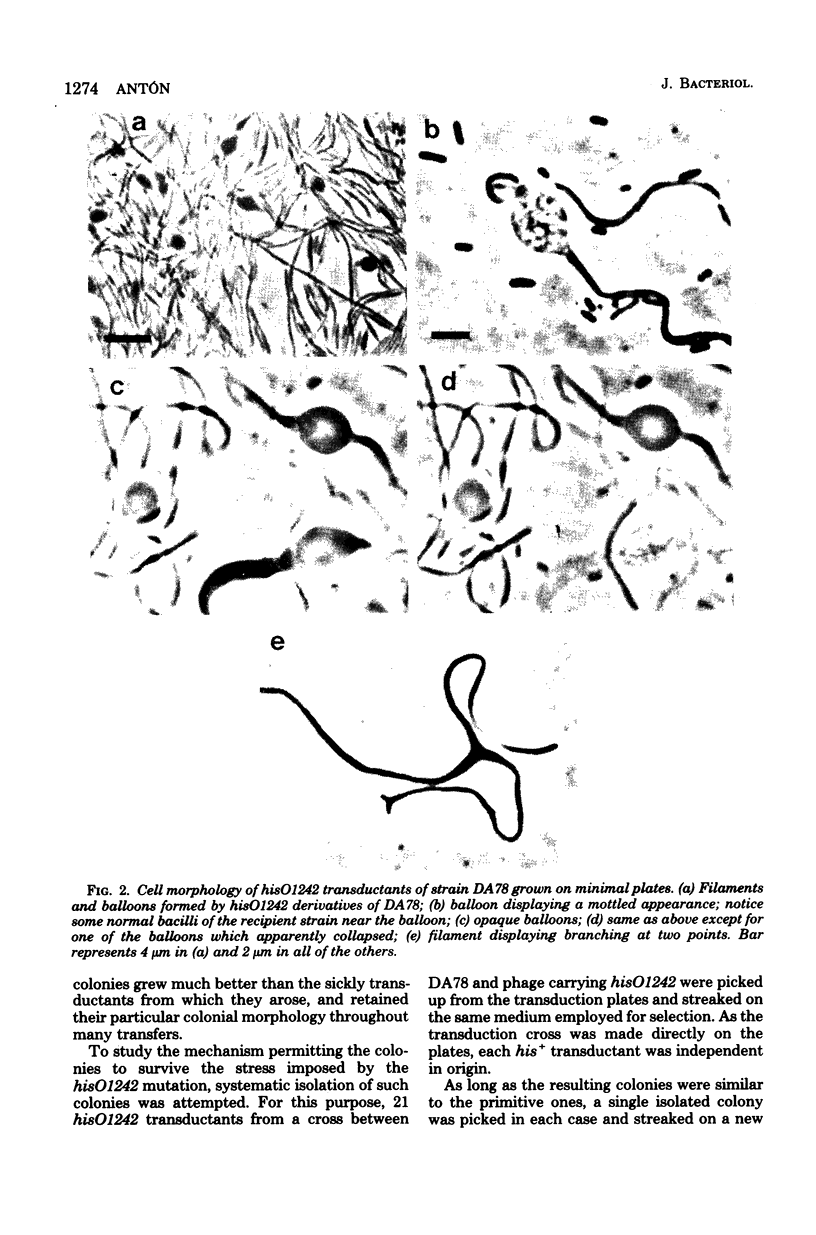
Strain SB564 and its derivative DA78 are hypersensitive to the inhibitory action of the proteins coded for by genes hisF and hisH on cell division. Transduction of hisO1243, a regulatory mutation that elicits a very high level of expression of the histidine operon, into these strains resulted in the production of long filamentous cells carrying large "balloons" and in growth failure. Forty-one hisO1242 derivatives that escaped inhibition were isolated. These strains showed a large variety of alterations, many of which were related to the cell envelope. The more-frequent alterations included: changes in cell shape, increased sensitivity to one or more of several drugs (deoxycholate, cycloserine, penicillin, novobiocin, acridine orange), increased autolytic activity in alkaline buffer, anomalous fermentation of maltose on eosin--methylene blue plates, and temperature-conditional cell division. The alterations are produced, in some of the strains, by pleiotropic mutations in gene envB (Antón, Mol, Gen. Genet. 160:277--286, 1978) or envD (Antón and Orce, Mol. Gen. Genet. 144:97--105, 1976). Strains affected in divC, divD, and rodA loci have also been identified. Genetic analysis has shown that several strains carry more than one envelope mutation. It is assumed that envelope mutations are positively selected because they somehow alleviate the particularly severe inhibition of cell division caused, in strains SB564 and DA78, by the excessive synthesis of hisF and hisH gene products.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antón D. N., Orce L. V. Envelope mutation promoting autolysis in Salmonella typhimurium. Mol Gen Genet. 1976 Feb 27;144(1):97–105. doi: 10.1007/BF00277311. [DOI] [PubMed] [Google Scholar]
- Cieśla Z., Bagdasarian M., Szczurkiewicz W., Przygońska M., Klopotowski T. Defective cell division in thermosensitive mutants of Salmonella typhimurium. Mol Gen Genet. 1972;116(2):107–125. doi: 10.1007/BF00582221. [DOI] [PubMed] [Google Scholar]
- Egan A. F., Russell R. R. Conditional mutations affecting the cell envelope of Escherichia coli K-12. Genet Res. 1973 Apr;21(2):139–152. doi: 10.1017/s001667230001332x. [DOI] [PubMed] [Google Scholar]
- Ely B., Fankhauser D. B., Hartman P. E. A fine structure map of the salmonella histidine operator-promoter. Genetics. 1974 Oct;78(2):607–631. doi: 10.1093/genetics/78.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely B. Physiological studies of salmonella histidine operator-promoter mutants. Genetics. 1974 Oct;78(2):593–606. doi: 10.1093/genetics/78.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink G. R., Klopotowski T., Ames B. N. Histidine regulatory mutants in Salmonella typhimurium. IV. A positive selection for polar histidine-requiring mutants from histidine operator constitutive mutants. J Mol Biol. 1967 Nov 28;30(1):81–95. doi: 10.1016/0022-2836(67)90245-8. [DOI] [PubMed] [Google Scholar]
- Hartman P. E., Hartman Z., Stahl R. C. Classification and mapping of spontaneous and induced mutations in the histidine operon of Salmonella. Adv Genet. 1971;16:1–34. doi: 10.1016/s0065-2660(08)60352-1. [DOI] [PubMed] [Google Scholar]
- Hartman P. E., Rusgis C., Stahl R. C. Orientation of the histidine operon in the Salmonella typhimurium linkage map. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1332–1335. doi: 10.1073/pnas.53.6.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaya M., Jones C. W., Khorana J., Strominger J. L. Mapping of the mecillinam-resistant, round morphological mutants of Escherichia coli. J Bacteriol. 1978 Jan;133(1):196–202. doi: 10.1128/jb.133.1.196-202.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates M. Bacterial lipids. Adv Lipid Res. 1964;2:17–90. [PubMed] [Google Scholar]
- Lugtenberg B., Peters R., Bernheimer H., Berendsen W. Influence of cultural conditions and mutations on the composition of the outer membrane proteins of Escherichia coli. Mol Gen Genet. 1976 Sep 23;147(3):251–262. doi: 10.1007/BF00582876. [DOI] [PubMed] [Google Scholar]
- Marr A. G., Ingraham J. L. EFFECT OF TEMPERATURE ON THE COMPOSITION OF FATTY ACIDS IN ESCHERICHIA COLI. J Bacteriol. 1962 Dec;84(6):1260–1267. doi: 10.1128/jb.84.6.1260-1267.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa H., Hayakawa K., Sato T., Imahori K. Characterization and genetic analysis of a mutant of Escherichia coli K-12 with rounded morphology. J Bacteriol. 1973 Jul;115(1):436–442. doi: 10.1128/jb.115.1.436-442.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. L., Hartman P. E. Overproduction of hisH and hisF gene products leads to inhibition of cell cell division in Salmonella. Can J Microbiol. 1972 May;18(5):671–681. doi: 10.1139/m72-105. [DOI] [PubMed] [Google Scholar]
- Nagel de Zwaig R., Luria S. E. Genetics and physiology of colicin-tolerant mutants of Escherichia coli. J Bacteriol. 1967 Oct;94(4):1112–1123. doi: 10.1128/jb.94.4.1112-1123.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J. R., Antón D. N., Hartman P. E. Histidine regulatory mutants in Salmonella typhimurium. I. Isolation and general properties. J Mol Biol. 1966 Dec 28;22(2):305–323. doi: 10.1016/0022-2836(66)90134-3. [DOI] [PubMed] [Google Scholar]
- Roth J. R., Hartman P. E. Heterogeneity in P22 transducing particles. Virology. 1965 Nov;27(3):297–307. doi: 10.1016/0042-6822(65)90109-1. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E., Hartman P. E. Linkage map of Salmonella typhimurium, edition V. Microbiol Rev. 1978 Jun;42(2):471–519. doi: 10.1128/mr.42.2.471-519.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savic D. J. A histidine operator-constitutive mutation decreases the frequency of recombination within the histidine operon of Salmonella typhimurium. Genetics. 1972 Jul;71(3):461–464. doi: 10.1093/genetics/71.3.461. [DOI] [PubMed] [Google Scholar]
- Steed P., Murray R. G. The cell wall and cell division of gram-negative bacteria. Can J Microbiol. 1966 Apr;12(2):263–270. doi: 10.1139/m66-036. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Voll M. J. Translation and polarity in the histidine operon. 3. The isolation of prototrophic polar mutations. J Mol Biol. 1967 Nov 28;30(1):109–124. doi: 10.1016/0022-2836(67)90247-1. [DOI] [PubMed] [Google Scholar]
- Weigand R. A., Rothfield L. I. Genetic and physiological classification of periplasmic-leaky mutants of Salmonella typhimurium. J Bacteriol. 1976 Jan;125(1):340–345. doi: 10.1128/jb.125.1.340-345.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westling-Häggström B., Normark S. Genetic and physiological analysis of an envB spherelike mutant of Escherichia coli K-12 and characterization of its transductants. J Bacteriol. 1975 Jul;123(1):75–82. doi: 10.1128/jb.123.1.75-82.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijsman H. J., Pafort H. C. Pleiotropic mutations in Escherichia coli conferring tolerance to glycine and sensitivity to penicillin. Mol Gen Genet. 1974;128(4):349–357. doi: 10.1007/BF00268522. [DOI] [PubMed] [Google Scholar]
- Wyche J. H., Kennedy J., Hartman Z., Hartman P. E., Diven J. Round-cell mutant of Salmonella typhimurium. J Bacteriol. 1974 Nov;120(2):965–969. doi: 10.1128/jb.120.2.965-969.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]