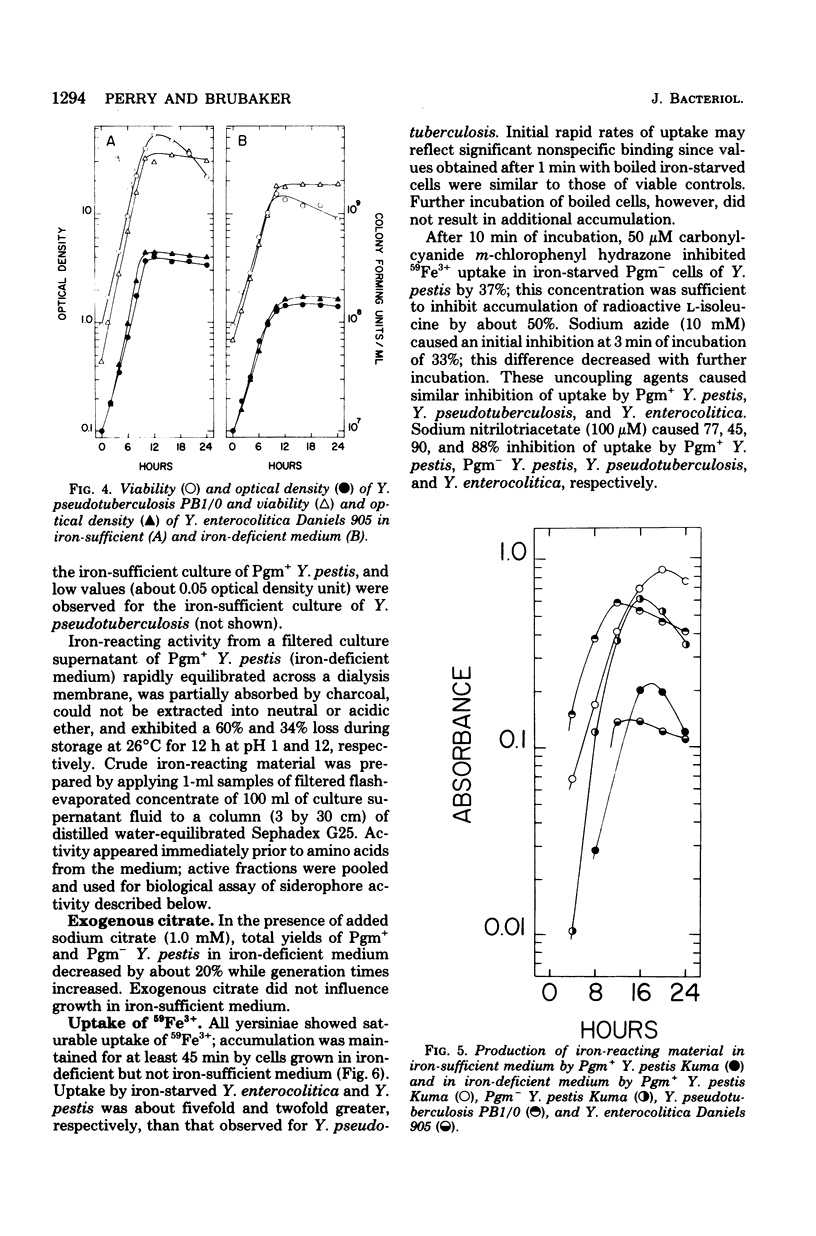
Abstract
Escherichia coli, Bacillus megaterium, and three species of yersiniae grew rapidly without significant production of soluble siderophores in a defined iron-sufficient medium (20 microM Fe3+). In iron-deficient medium (0.1 to 0.3 microM Fe3+) all organisms showed reduced growth, and there was extensive production of siderophores by E. coli and B. megaterium. Release of soluble siderophores by Yersinia pestis, Y. pseudotuberculosis, or Y. enterocolitica in this medium was not detected. Citrate (1 mM) inhibited growth of yersiniae in iron-deficient medium, indicating that the organisms lack an inducible Fe3+-citrate transport mechanism. Uptake of 59Fe3+ by all yersiniae was an energy-dependent saturable process, showing increased accumulation after adaptation to iron-deficient medium. Growth of Y. pseudotuberculosis and Y. enterocolitica but not Y. pestis on iron-limited solid medium was enhanced to varying degrees by exogenous siderophores (desferal, schizokinen, aerobactin, and enterochelin). Only hemin (0.1 pmol) or a combination of inorganic iron plus protoporphyrin IX promoted growth of Y. pestis on agar rendered highly iron deficient with egg white conalbumin (10 microM). Growth of Y. pseudotuberculosis and Y. enterocolitica was stimulated on this medium by Fe3+ or hemin. These results indicate that hemin can serve as a sole source of iron for yersiniae and that the organisms possess an efficient cell-bound transport system for Fe3+.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURROWS T. W., JACKSON S. The pigmentation of Pasteurella pestis on a defined medium containing haemin. Br J Exp Pathol. 1956 Dec;37(6):570–576. [PMC free article] [PubMed] [Google Scholar]
- BURROWS T. W., JACKSON S. The virulence-enhancing effect of iron on nonpigmented mutants of virulent strains of Pasteurella pestis. Br J Exp Pathol. 1956 Dec;37(6):577–583. [PMC free article] [PubMed] [Google Scholar]
- Brubaker R. R. The genus Yersinia: biochemistry and genetics of virulence. Curr Top Microbiol Immunol. 1972;57:111–158. doi: 10.1007/978-3-642-65297-4_4. [DOI] [PubMed] [Google Scholar]
- Frost G. E., Rosenberg H. The inducible citrate-dependent iron transport system in Escherichia coli K12. Biochim Biophys Acta. 1973 Nov 30;330(1):90–101. doi: 10.1016/0005-2736(73)90287-3. [DOI] [PubMed] [Google Scholar]
- Garibaldi J. A. Influence of temperature on the biosynthesis of iron transport compounds by Salmonella typhimurium. J Bacteriol. 1972 Apr;110(1):262–265. doi: 10.1128/jb.110.1.262-265.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garibaldi J. A. Influence of temperature on the iron metabolism of a fluorescent pseudomonad. J Bacteriol. 1971 Mar;105(3):1036–1038. doi: 10.1128/jb.105.3.1036-1038.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson F., Magrath D. I. The isolation and characterization of a hydroxamic acid (aerobactin) formed by Aerobacter aerogenes 62-I. Biochim Biophys Acta. 1969 Nov 18;192(2):175–184. doi: 10.1016/0304-4165(69)90353-5. [DOI] [PubMed] [Google Scholar]
- Kingdon G. C., Sword C. P. Effects of Listeria monocytogenes Hemolysin on Phagocytic Cells and Lysosomes. Infect Immun. 1970 Apr;1(4):356–362. doi: 10.1128/iai.1.4.356-362.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochan I., Kvach J. T., Wiles T. I. Virulence-associated acquisition of iron in mammalian serum by Escherichia coli. J Infect Dis. 1977 Apr;135(4):623–632. doi: 10.1093/infdis/135.4.623. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leong J., Neilands J. B. Mechanisms of siderophore iron transport in enteric bacteria. J Bacteriol. 1976 May;126(2):823–830. doi: 10.1128/jb.126.2.823-830.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckey M., Pollack J. R., Wayne R., Ames B. N., Neilands J. B. Iron uptake in Salmonella typhimurium: utilization of exogenous siderochromes as iron carriers. J Bacteriol. 1972 Sep;111(3):731–738. doi: 10.1128/jb.111.3.731-738.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macham L. P., Ratledge C. A new group of water-soluble iron-binding compounds from Mycobacteria: the exochelins. J Gen Microbiol. 1975 Aug;89(2):379–382. doi: 10.1099/00221287-89-2-379. [DOI] [PubMed] [Google Scholar]
- Macham L. P., Ratledge C., Nocton J. C. Extracellular iron acquisition by mycobacteria: role of the exochelins and evidence against the participation of mycobactin. Infect Immun. 1975 Dec;12(6):1242–1251. doi: 10.1128/iai.12.6.1242-1251.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers W. F., Osterman J. V., Wisseman C. L., Jr Nutritional studies of Rickettsia guintana: nature of the hematin requirement. J Bacteriol. 1972 Jan;109(1):89–95. doi: 10.1128/jb.109.1.89-95.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien I. G., Gibson F. The structure of enterochelin and related 2,3-dihydroxy-N-benzoylserine conjugates from Escherichia coli. Biochim Biophys Acta. 1970 Aug 14;215(2):393–402. doi: 10.1016/0304-4165(70)90038-3. [DOI] [PubMed] [Google Scholar]
- Pollack J. R., Ames B. N., Neilands J. B. Iron transport in Salmonella typhimurium: mutants blocked in the biosynthesis of enterobactin. J Bacteriol. 1970 Nov;104(2):635–639. doi: 10.1128/jb.104.2.635-639.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]




