Abstract
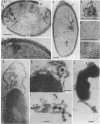
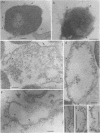
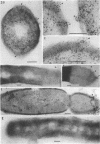
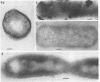
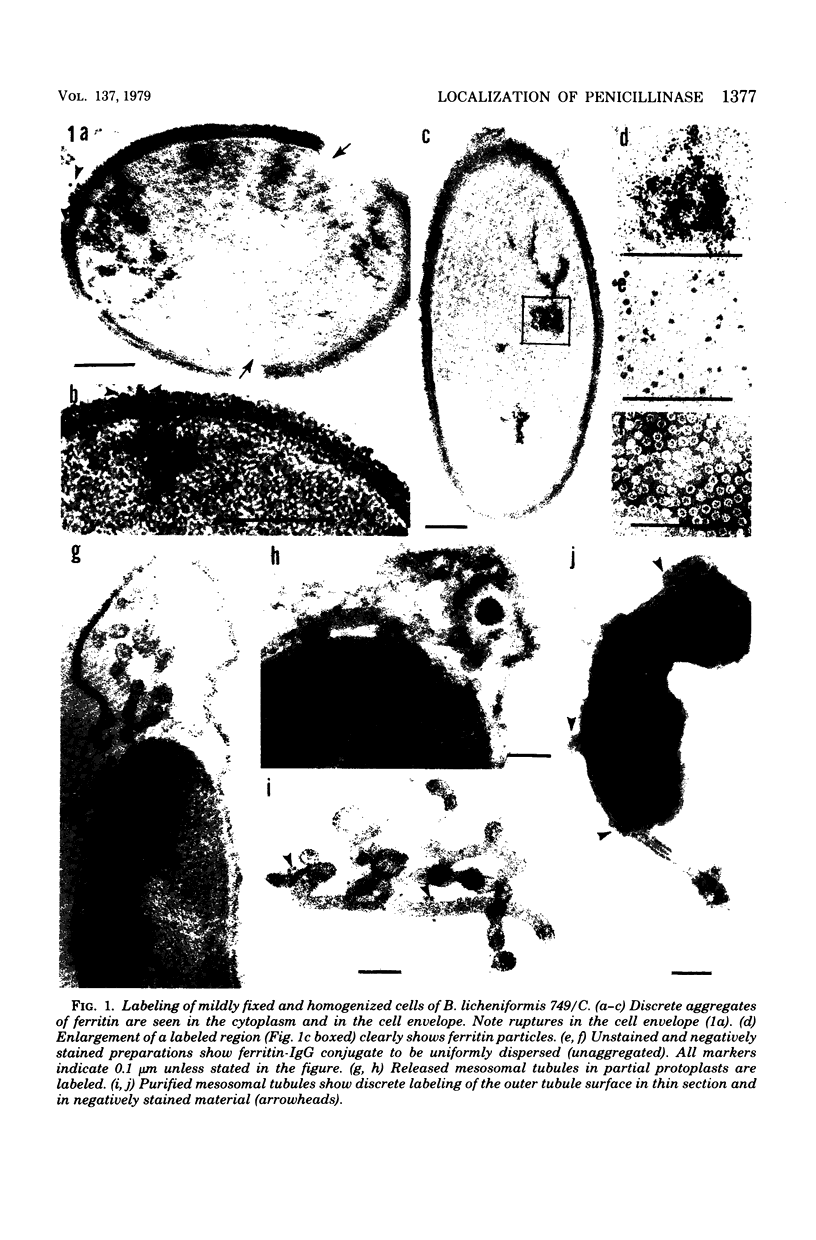
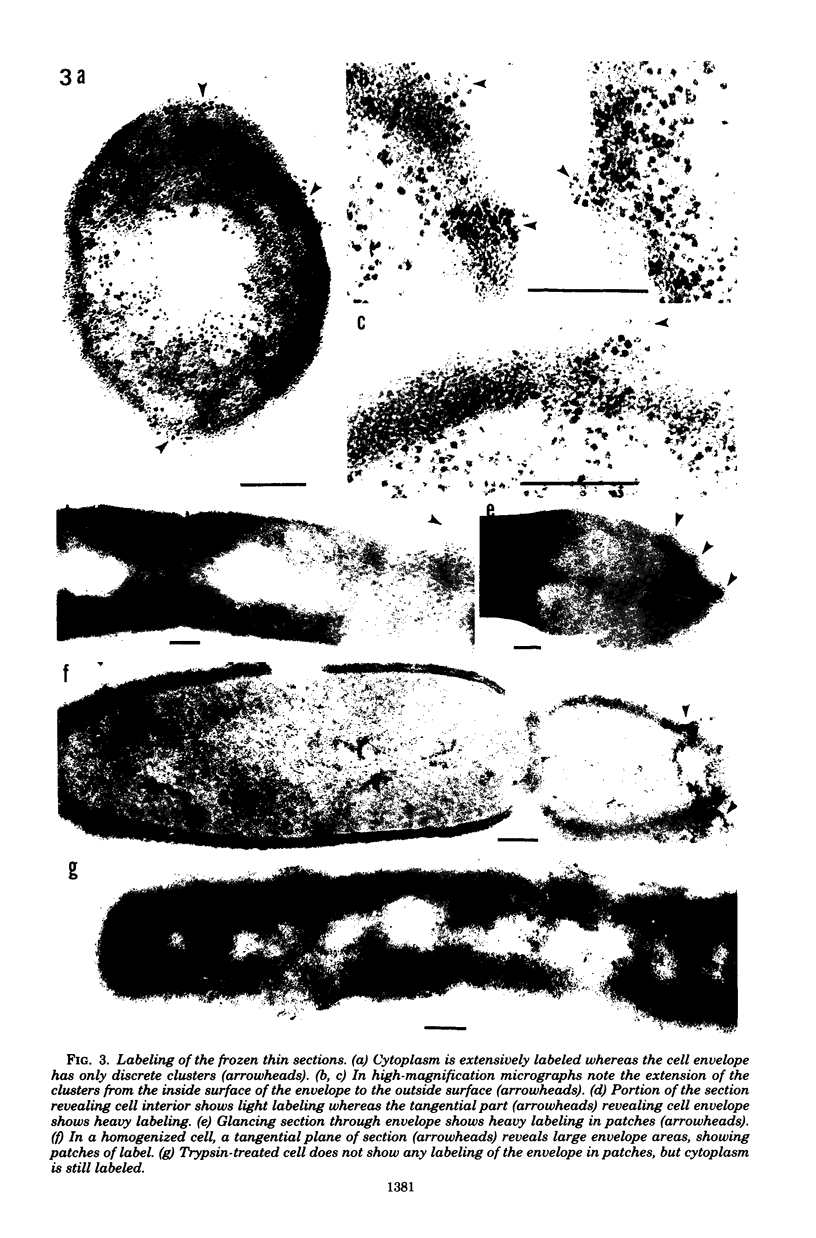
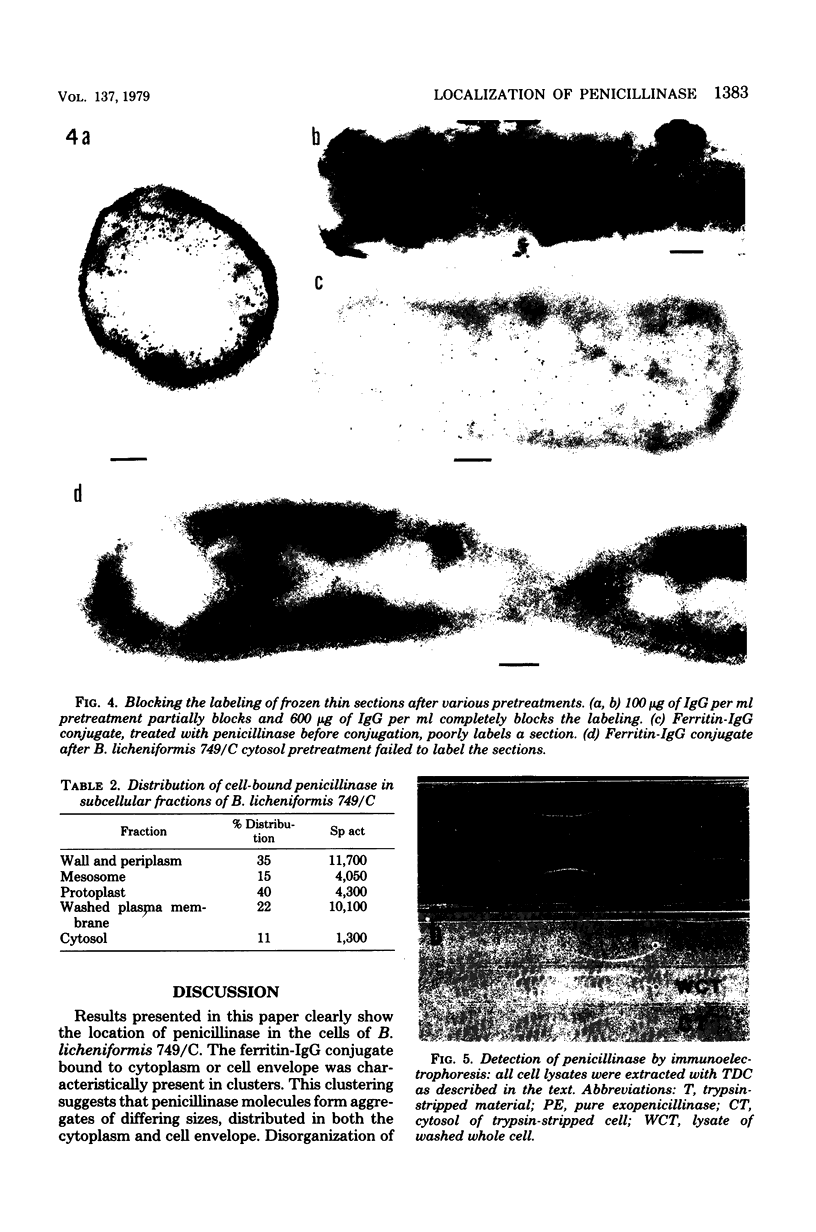
Penicillinase was localized in log-phase cells of Bacillus licheniformis 749/C by labeling with ferritin-anti-penicillinase immunoglobulin G conjugate. Mildly fixed homogenized cells, isolated subcellular fractions, and frozen thin sections were labeled. The label was distributed in discrete patches in the cell envelope. The patches extended from the inside part of the membrane to the outside part of the wall. The inside part of the membrane was labeled more extensively than the outside part. The cytoplasm also bound some ferritin-immunoglobulin G conjugate. Immunoelectrophoresis and biochemical assay of cytosol material suggest that the cytoplasmic antigenic sites are a protease-sensitive form of penicillinase.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiyappa P. S., Traficante L. J., Lampen J. O. Penicillinase-releasing protease of Bacillus licheniformis: purification and general properties. J Bacteriol. 1977 Jan;129(1):191–197. doi: 10.1128/jb.129.1.191-197.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettinger G. E., Lampen J. O. Further evidence for a partially folded intermediate in penicillinase secretion by Bacillus licheniformis. J Bacteriol. 1975 Jan;121(1):83–90. doi: 10.1128/jb.121.1.83-90.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., McInnes J. L., Hanlon J. E., May B. K., Elliott W. H. Evidence for an accumulation of messenger RNA specific for extracellular protease and its relevance to the mechanism of enzyme secretion in bacteria. J Mol Biol. 1972 Jun 20;67(2):199–217. doi: 10.1016/0022-2836(72)90236-7. [DOI] [PubMed] [Google Scholar]
- Cancedda R., Schlesinger M. J. Localization of polyribosomes containing alkaline phosphatase nascent polypeptides on membranes of Escherichia coli. J Bacteriol. 1974 Jan;117(1):290–301. doi: 10.1128/jb.117.1.290-301.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen A. K. Frozen thin sections of fresh tissue for electron microscopy, with a description of pancreas and liver. J Cell Biol. 1971 Dec;51(3):772–804. doi: 10.1083/jcb.51.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh B. K., Carroll K. K. Isolation, composition, and structure of membrane of Listeria monocytogenes. J Bacteriol. 1968 Feb;95(2):688–699. doi: 10.1128/jb.95.2.688-699.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh B. K. Grooves in the plasmalemma of Saccharomyces cerevisiae seen in glancing sections of double aldehyde-fixed cells. J Cell Biol. 1971 Jan;48(1):192–197. doi: 10.1083/jcb.48.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh B. K., Sargent M. G., Lampen J. O. Morphological phenomena associated with penicillinase induction and secretion in Bacillus licheniformis. J Bacteriol. 1968 Oct;96(4):1314–1328. doi: 10.1128/jb.96.4.1314-1328.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh B. K. The mesosome--a clue to the evolution of the plasma membrane. Subcell Biochem. 1974 Dec;3(4):311–367. [PubMed] [Google Scholar]
- Inouye H., Beckwith J. Synthesis and processing of an Escherichia coli alkaline phosphatase precursor in vitro. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1440–1444. doi: 10.1073/pnas.74.4.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida Y., Olsen B. R., Berg R. A., Prockop D. J. Two improved methods for preparing ferritin-protein conjugates for electron microscopy. J Cell Biol. 1975 Feb;64(2):331–339. doi: 10.1083/jcb.64.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreibich G., Ulrich B. L., Sabatini D. D. Proteins of rough microsomal membranes related to ribosome binding. I. Identification of ribophorins I and II, membrane proteins characteristics of rough microsomes. J Cell Biol. 1978 May;77(2):464–487. doi: 10.1083/jcb.77.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampen J. O. Movement of extracellular enzymes across cell membranes. Symp Soc Exp Biol. 1974;(28):351–374. [PubMed] [Google Scholar]
- Nagata Y., Yamaguchi K., Maruo B. Genetic and biochemical studies on cell-bound alpha-amylase in Bacillus subtilis Marburg. J Bacteriol. 1974 Aug;119(2):425–430. doi: 10.1128/jb.119.2.425-430.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLLOCK M. R. PURIFICATION AND PROPERTIES OF PENICILLINASES FROM TWO STRAINS OF BACILLUS LICHENIFORMIS: A CHEMICAL, PHYSICOCHEMICAL AND PHYSIOLOGICAL COMPARISON. Biochem J. 1965 Mar;94:666–675. doi: 10.1042/bj0940666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E., Lenard J. Membrane asymmetry. Science. 1977 Feb 25;195(4280):743–753. doi: 10.1126/science.402030. [DOI] [PubMed] [Google Scholar]
- Sargent M. G., Ghosh B. K., Lampen J. O. Localization of cell-bound penicillinase in Bacillus licheniformis. J Bacteriol. 1968 Oct;96(4):1329–1338. doi: 10.1128/jb.96.4.1329-1338.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. G., Lampen J. O. A mechanism for penicillinasesecretion in Bacillus licheniformis. Proc Natl Acad Sci U S A. 1970 Apr;65(4):962–969. doi: 10.1073/pnas.65.4.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. G., Lampen J. O. Organization of the membrane-bound penicillinases of Bacillus licheniformis. Arch Biochem Biophys. 1970 Jan;136(1):167–177. doi: 10.1016/0003-9861(70)90338-3. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu K. T., Singer S. J. Improved procedures for immunoferritin labeling of ultrathin frozen sections. J Cell Biol. 1976 Dec;71(3):894–906. doi: 10.1083/jcb.71.3.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traficante L. J., Lampen J. O. Vesicle penicillinase of Bacillus licheniformis: existence of periplasmic-releasing factor(s). J Bacteriol. 1977 Jan;129(1):184–190. doi: 10.1128/jb.129.1.184-190.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]