Abstract
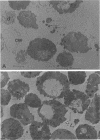
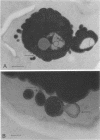
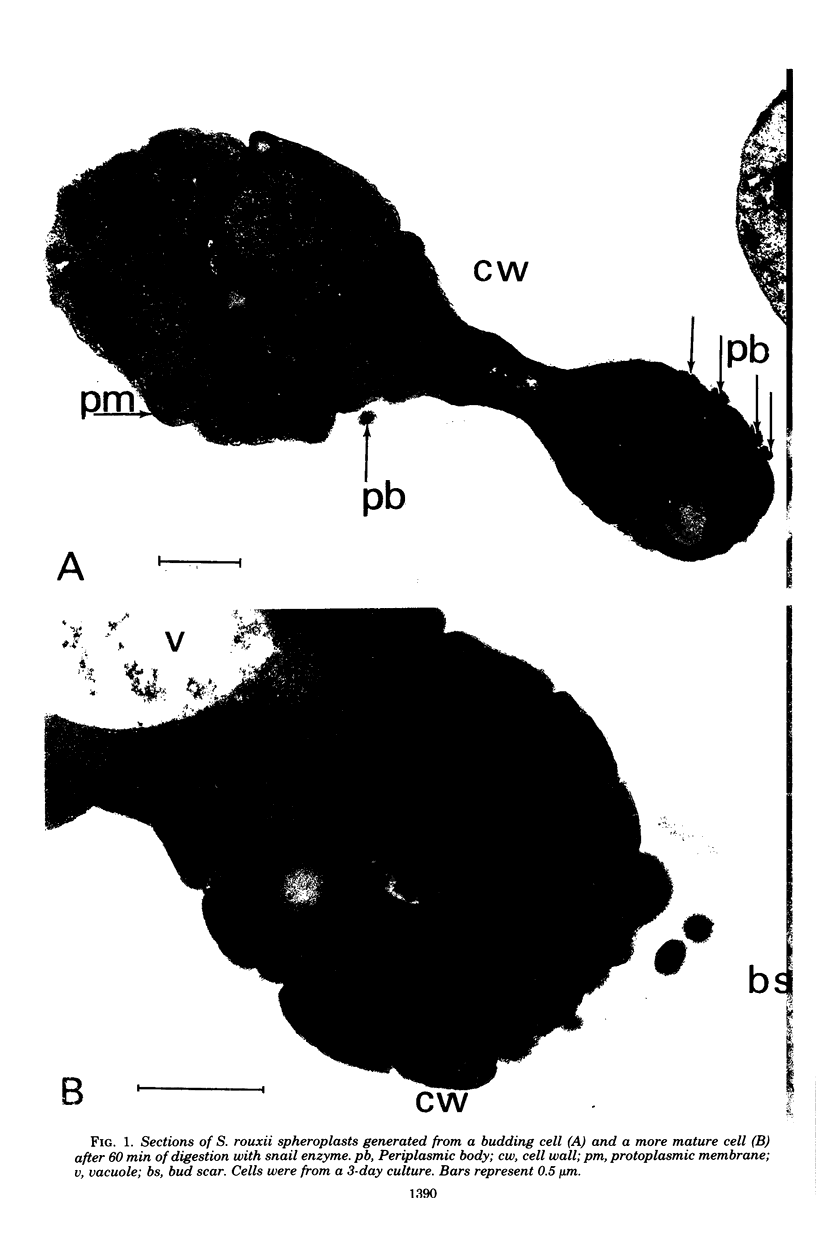
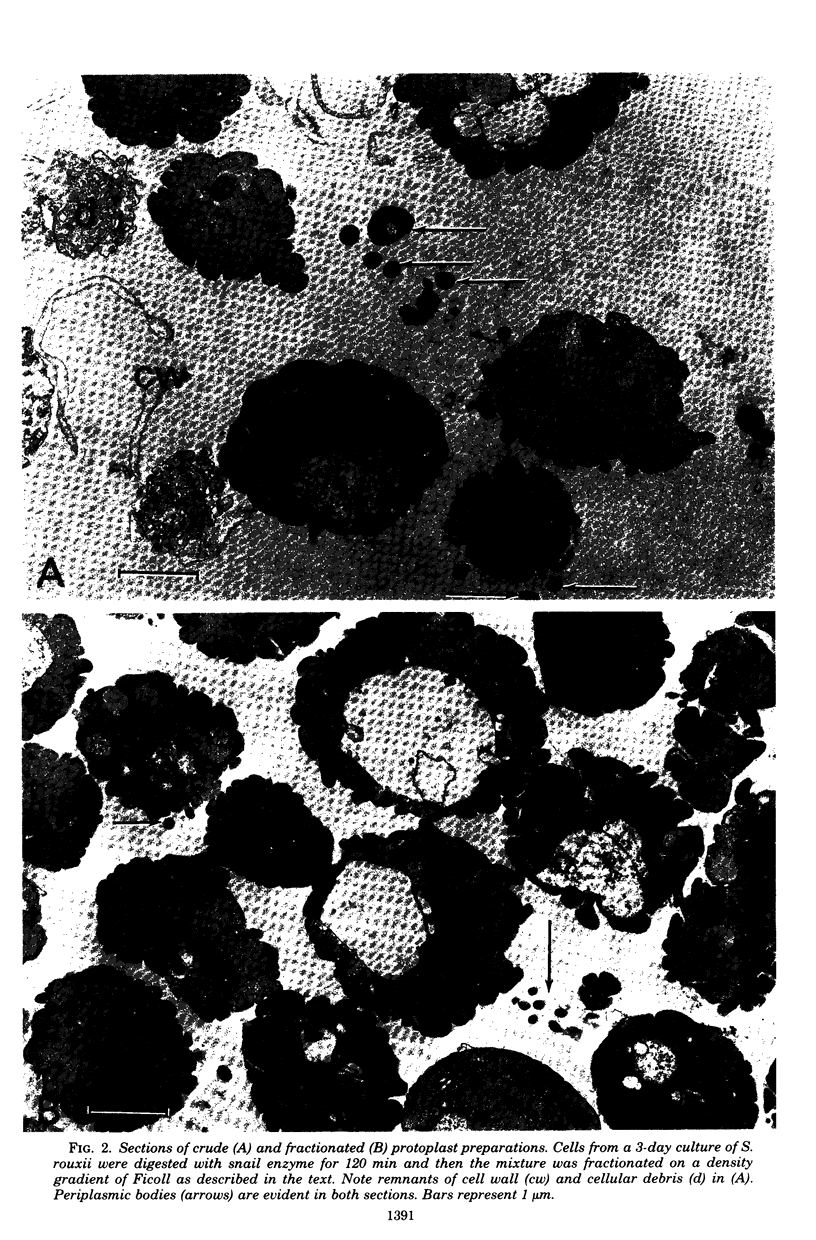
Cells of the osmotolerant yeast Saccharomyces rouxii were transformed to protoplasts in good yield (85%) by digesting cell walls with snail-gut enzyme in the presence of 10 mM dithioerythritol, 0.1 M sodium phosphate buffer (pH 6.8), and 2.0 M KCl. The requirement for 2.0 M KCl compares with that for S. bisporus var. mellis (another osmotolerant species) and contrasts with the 0.3 to 0.8 M KCl concentrations used in the preparation of most yeast protoplasts. Short digestions (60 min or less) produced mostly spheroplasts; longer incubations (90 min or more) yielded mostly protoplasts as judged by electron micrographs. These protoplasts could be transferred to 1.0 M KCl or 2.0 M sorbitol without lysing, but lysis was pronounced in 0.5 M KCl or 1.0 M mannitol and complete in 0.02 M KCl. Protoplasts were separated from isolated cell wall remnants and debris by centrifugation on a linear gradient of Ficoll 400 (35 to 17.5%, wt/vol) containing 2.0 M KCl. Both crude and fractionated protoplast preparations contained vesicles which were identified with the periplasmic bodies of whole cells. Some of the periplasmic bodies were connected to protoplasts by fine pedicels; others appeared free. Independent degeneracy of periplasmic bodies was occasionally observed. β-Fructofuranosidase (EC 3.2.1.26) activity is cryptic (physically) in cells of S. rouxii in contrast to the expressed enzyme (periplasmic space) of other Saccharomyces species. This enzyme remains cryptic in protoplast preparations of S. rouxii but is expressed upon lysis. The same specific activities were found per unit cell or protoplast. The possible association of the cryptic enzyme with periplasmic bodies is discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold W. N. Expression of cryptic beta-fructofuranosidase in Saccharomyces rouxii. J Bacteriol. 1974 Nov;120(2):886–894. doi: 10.1128/jb.120.2.886-894.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold W. N., Garrison R. G., Boyd S. K. Periplasmic structure in Saccharomyces rouxii (Boutroux), an osmophil. Appl Microbiol. 1974 Dec;28(6):1047–1054. doi: 10.1128/am.28.6.1047-1054.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold W. N., Lacy J. S. Permeability of the cell envelope and osmotic behavior in Saccharomyces cerevisiae. J Bacteriol. 1977 Aug;131(2):564–571. doi: 10.1128/jb.131.2.564-571.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold W. N. Location of acid phosphatase and -fructofuranosidase within yeast cell envelopes. J Bacteriol. 1972 Dec;112(3):1346–1352. doi: 10.1128/jb.112.3.1346-1352.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestic P. B., Arnold W. N. Linear transformation of standard curves for yeast turbidity. Appl Environ Microbiol. 1976 Oct;32(4):640–641. doi: 10.1128/aem.32.4.640-641.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUELL E. A., INOUE S., UTTER M. F. ISOLATION AND PROPERTIES OF INTACT MITOCHONDRIA FROM SPHEROPLASTS OF YEAST. J Bacteriol. 1964 Dec;88:1762–1773. doi: 10.1128/jb.88.6.1762-1773.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling S., Theilade J., Birch-Andersen A. Kinetic and morphological observations on Saccharomyces cerevisiae during spheroplast formation. J Bacteriol. 1969 May;98(2):797–810. doi: 10.1128/jb.98.2.797-810.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecká M., Svoboda A., Brichta J. Effect of "osmotic stabilizers" and glycerol on yeast cell envelopes. Z Allg Mikrobiol. 1973;13(6):481–487. doi: 10.1002/jobm.3630130605. [DOI] [PubMed] [Google Scholar]
- Mann J. W., Heintz C. E., Macmillan J. D. Yeast spheroplasts formed by cell wall-degrading enzymes from Oerskovia sp. J Bacteriol. 1972 Sep;111(3):821–824. doi: 10.1128/jb.111.3.821-824.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. J., Grand R. J. Comparative studies on beta-glucan hydrolases. Isolation and characterization of an exo(1 yields 3)-beta-glucanase from the snail, Helix pomatia. Arch Biochem Biophys. 1975 Mar;167(1):165–175. doi: 10.1016/0003-9861(75)90453-1. [DOI] [PubMed] [Google Scholar]
- ONISHI H. OSMOPHILIC YEASTS. Adv Food Res. 1963;12:53–94. [PubMed] [Google Scholar]
- PAPPAGIANIS D., PHAFF H. J. Delayed fermentation of sucrose by certain haploid species of Saccharomyces. Antonie Van Leeuwenhoek. 1956;22(4):353–370. doi: 10.1007/BF02538349. [DOI] [PubMed] [Google Scholar]
- Weimberg R., Orton W. L. Elution of Acid Phosphatase from the Cell Surface of Saccharomyces mellis by Potassium Chloride. J Bacteriol. 1965 Jul;90(1):82–94. doi: 10.1128/jb.90.1.82-94.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]