Abstract
Allograft rejection is a process of immune reactivity triggered by foreign transplantation antigens. We now demonstrate that the 60-kDa heat shock protein (hsp60), a molecule that is identical in the donor and the recipient, can regulate allograft immunity. In wild-type mice, hsp60 expression was greatly enhanced in allografts being rejected. By using MHC class II (Eα) promoter hsp60 transgenic mice either as donors of skin with enhanced expression of hsp60, or as allograft recipients with decreased hsp60 autoimmunity, we found that augmented expression of mouse hsp60 in the allograft accelerated its rejection, whereas reduced autoimmunity to mouse hsp60 in graft recipients delayed the process. Moreover, in nontransgenic mice, therapeutic administration of hsp60 or hsp60 peptides, known to modulate naturally occurring hsp60 autoimmunity, led to delayed allograft rejection. Thus, we demonstrate that hsp60 expression and hsp60 autoimmunity can influence and modify the immune response to foreign antigens. Hence, autoimmunity to self-hsp60 epitopes is not necessarily an aberration, but may serve physiologically and therapeutically to modulate foreign immunity.
Autoimmunity to the 60-kDa heat shock protein (hsp60) has gained notoriety for its apparent involvement in tissue-specific autoimmune diseases such as type I diabetes (1–7), arthritis (7–9), and encephalomyelitis (10, 11). However, hsp60 autoimmunity is also prevalent in healthy individuals free of immune inflammatory disease (12–14). It has been unclear whether this autoimmunity is simply an unnecessary remnant of evolution, or whether it may have a functional role. We now demonstrate that hsp60 autoimmunity is involved in the rejection of unwanted cells. These findings suggest that autoimmunity is not a mere aberration, but could have a functional role in preserving health. Moreover, we show that therapeutic modulation of hsp60 autoimmunity can alter the course of allograft rejection, a process that has been classically regarded as nonautoimmune.
MATERIALS AND METHODS
Western Blot Analysis.
Shaved skin was removed surgically, as described below, and lysed in TENN buffer (50 mM Tris, pH 8.0/5 mM EDTA/0.5% NP-40/150 mM NaCl). Equal amounts of protein (50 μg, determined by using the Bradford reagent, Bio-Rad) were fractionated on 10% SDS/PAGE and transferred to nitrocellulose paper (15) for Western blot analysis using LK1 (a gift from Willem van Eden, Utrecht, The Netherlands), a mAb specific for hsp60 (16) at a 1:500 dilution, and the ECL detection system (Amersham Pharmacia).
Spleen T Cell Proliferation Assay.
Spleen cell suspensions obtained from 2-month-old NOD mice were assayed as described (5). Briefly, splenocytes, 2 × 105/ml, were incubated in triplicate for 72 h in 0.2 ml of culture medium in microtiter wells in the presence or absence of various antigens at 5 μg/ml. Proliferation was measured by the incorporation of [3H]thymidine into DNA during the final 12 h of incubation.
Skin Transplantation.
Sections of skin (1 cm2) were cut from the backs of sacrificed donor mice and washed in saline. Patches of dorsal skin of 1 cm2 were removed from anesthetized recipient mice (treated with Nembutal 6 mg/ml, 0.25 ml per mouse) and replaced by the donor skin grafts. Histoacryl (Braun, Melsungen, Germany) was applied around the grafts, and Nobecutan (ASTR, Astra Tech, Glos, United Kingdom) was sprayed over them. Statistical analysis of skin graft survival was done by using the Wilcoxon test.
Peptides and Protein.
Peptides of the mouse hsp60 sequence were synthesized and purified as described (2). The sequence of peptide p11 is VIAELKKQSKPVTTPEEIAQ and that of peptide p12 is EEIAQVATISANGDKDIGNI (17). Peptide p277 is the V-substituted human p277 peptide VLGGGVALLRVIPALDSLTPANED as described (2) and shown to be crossreactive with the mouse p277 homologue (5). The mycobacterial hsp60 peptide MT-p278, which is a T cell epitope in NOD mice (18), is EGDEATGANIVKVALEA. p278 is the mouse homologue of MT-p278, NEDQKIGIEIIKRALKI, which is not a T cell epitope in NOD mice. Recombinant full-length hsp60 was prepared as described (18).
Reverse Transcription-PCR and Sequencing.
The hsp60 cDNA was amplified by PCR from NOD spleen cDNA in two fragments spanning the whole coding region (VENT proof-reading Taq polymerase). The two fragments then were cloned by using the TA vector into plasmid PGEM T EASY (Promega) and sequenced by using T3 and T7 primers (Alfexpress, Amersham Pharmacia).
RESULTS
Augmented Expression of hsp60 in Rejected Allografts.
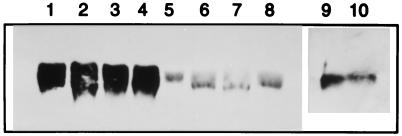
Fig. 1 shows the expression of mouse hsp60 in wild-type mouse skin transplanted to allogeneic wild-type hosts and undergoing rejection (lanes 1–4), or transplanted to syngeneic hosts and tolerated (lanes 5–8). It can be seen that the process of rejection augments the expression of hsp60 in wild-type skin (compare lanes 1–4 to 5–8).
Figure 1.
Western blot analysis of hsp60 expression in mouse skin. Lanes 1–4 show hsp60 expression during rejection of BALB/c (H-2d) skin removed 10 days after transplantation to allogeneic NOD (H-2g7) mice. Lanes 5–8 show the expression of hsp60 in BALB/c skin transplanted 10 days earlier to syngeneic BALB/c mice. Lane 9 shows the spontaneous expression of hsp60 in untransplanted Eα.hsp60 transgenic NOD skin. Lane 10 shows the expression of hsp60 in untransplanted wild-type NOD skin.
Hsp60 Overexpression in the Allograft Enhances its Rejection.
To test whether the enhanced local expression of hsp60 noted above has a functional role in the rejection process, we used transgenic NOD mice hyperexpressing mouse hsp60 under the direction of the promoter of the MHC class II Eα molecule (19). Fig. 1 shows that the expression of hsp60 is constitutively augmented in the skin of Eα.hsp60 transgenic NOD mice (lane 9) compared with that in the skin of wild-type NOD mice (lane 10), probably because of activation of the MHC class II promoter in skin dendritic cells (20). The consequences of this constitutive hyperexpression are shown in Fig. 2.
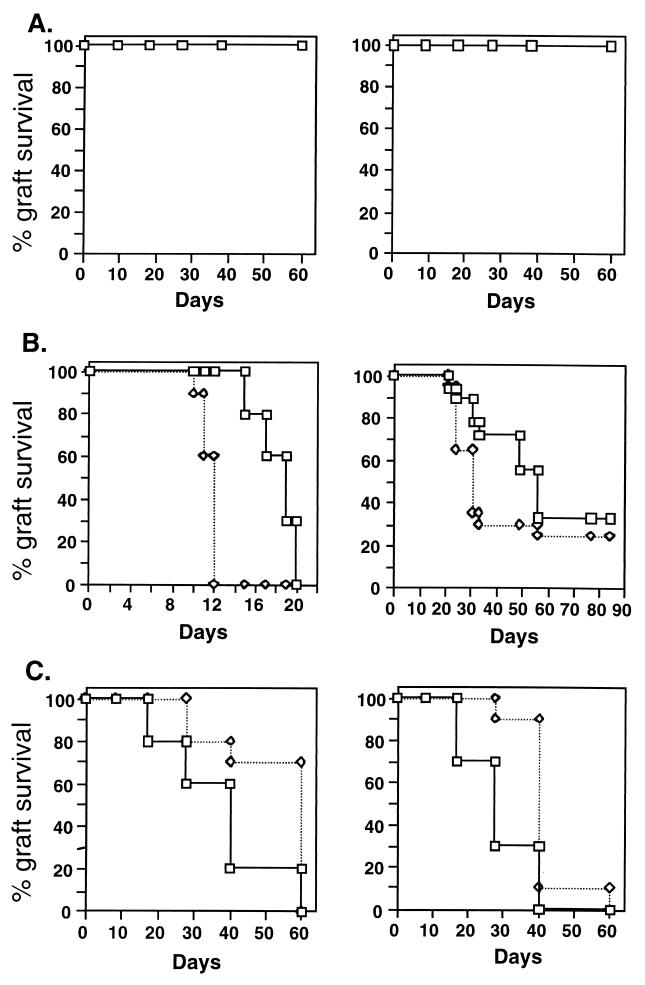
Figure 2.
Expression of hsp60 affects the rejection of skin allografts. (A, Left) Eα.hsp60 transgenic skin is not rejected when transplanted to wild-type NOD mice. (A, Right) Eα.hsp60 transgenic mice do not reject wild-type skin. (B, Left) The accelerated rejection (P < 0.0001) of Eα.hsp60 transgenic NOD skin (◊) compared with wild-type NOD skin (□) transplanted to BALB/c mice. (B, Right) The accelerated rejection (P < 0.038) of Eα.hsp60 transgenic male NOD skin transplanted to HY incompatible, wild-type female NOD mice. (C, Left) The delay in rejection (P = 0.004) of allogeneic C57BL/6 (H-2b) skin by Eα.hsp60 transgenic NOD mice (H-2g7). (C, Right) The delay in rejection (P = 0.001) of BALB/k (H-2k) skin by Eα.hsp60 transgenic mice. Each group contained 10–12 mice. ◊, Eα.hsp60 recipients; □, control wild-type mice. The mice used in these and other experiments in the paper were 2 months old.
Fig. 2A shows that the Eα.hsp60 NOD transgenic mice and nontransgenic NOD mice do not mutually reject skin. Thus, the hsp60 transgenic skin has not acquired a foreign histocompatibility antigen during insertion of the mouse hsp60 transgene. To confirm this at the molecular level, we cloned and sequenced the NOD hsp60 gene and found it to be identical in sequence to the hsp60 molecule used in the generation of the transgenic mice (not shown). Thus, the Eα.hsp60 transgenic skin is still self. Nevertheless, Fig. 2B shows that the overexpression of hsp60 in the transgenic skin can accelerate the allograft rejection in two different rejection systems. Fig. 2B, Left shows acceleration of skin rejection across the MHC barrier (NOD H-2g7 skin transplanted to the partially allogeneic BALB/c H-2d mice, which share the K locus, but differ at the I-A and D loci), the median day of rejection being reduced from 20 to 12 days (P = 0.0001). Fig. 2B, Right shows accelerated rejection by female NOD recipients of male Eα.hsp60 transgenic NOD skin compared with rejection of wild-type male skin, the median day of rejection being reduced from 55 days to 30 days (P = 0.038). The rejection of male skin by syngeneic female mice is caused by a minor histocompatibility antigen encoded by the male Y chromosome (21). Thus, the constitutive hyperexpression of self-hsp60 in donor transgenic skin accelerates the rejection reaction, a reaction triggered by foreign transplantation antigens, either major or minor.
Depressed Host Autoimmunity to hsp60 Attenuates Allograft Rejection.
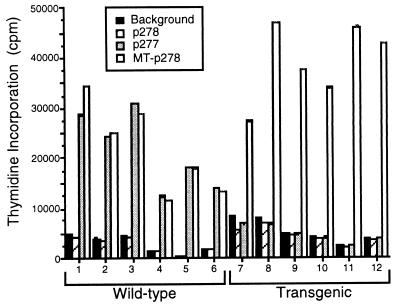
Allelic polymorphism of mouse hsp60 has not been reported; the hsp60 molecules of various mouse strains have been cloned and were found to be identical in sequence (10, 22). In other words, the hsp60 molecule in the above experiments was identical in donor and recipient. Thus, it is conceivable that hsp60 autoimmunity mediates the enhancement of the rejection process that ensues after augmented expression of hsp60 in the graft. To explore this possibility, we again used the Eα.hsp60 transgenic mice, only this time as allograft recipients rather than as donors. Regular NOD mice express hsp60 only in the medulla of the thymus (19) and develop T cells targeted at their self hsp60 (1, 2, 5, 19); the transgenic mice, in contrast, express large amounts of self hsp60 in the thymic medulla and in the thymic cortex (19) and are practically devoid of spontaneous T cell reactivity to self hsp60 epitopes, such as peptide p277 (Fig. 3). This loss of spontaneous T cell reactivity in the transgenics is specific to hsp60 autoimmunity, as the T cell responses of the transgenics to epitopes of other molecules, such as mycobacterial hsp60 peptide MT-p278, remain unaltered (Fig. 3). In Fig. 2C, the recipient NOD mice are bearers of the Eα.hsp60 transgene. Compared with wild-type NOD mice, the Eα.hsp60 transgenic mice manifested a delayed median day of rejection of allogeneic C57BL/6 (H-2b) skin grafts (Fig. 2C, Left) by 20 days (P = 0.004) and of BALB.K (H-2k) skin grafts (Fig. 2C , Right panel) by 10 days (P = 0.0001). Rejections of BALB/c (H-2d) and BALB.B (H-2b) skins were also significantly delayed (P = 0.001 and P = 0.015, respectively, not shown). Thus, the transgenic recipients, which manifest attenuated hsp60 autoimmunity, mount a slower allograft rejection reaction.
Figure 3.
Spontaneous T cell proliferation of wild-type or hsp60-transgenic NOD mice to hsp60 peptides. Groups of six 2-month-old mice were tested individually for spontaneous reactivity to hsp60 peptides p278, p277, and MT-p278, and to no added peptide (control). Compared with wild-type NOD mice, the Eα.hsp60 NOD transgenics manifest a lack of spontaneous T cell reactivity to the self-hsp60 peptide p277, but their spontaneous T cell reactivity to a foreign T-cell epitope in NOD mice (18), the mycobacterial hsp60 peptide MT-p278, is not diminished. The mouse homologue of MT-p278, p278, is not a T cell epitope in NOD mice.
Vaccination with hsp60 and hsp60 Peptides Affects Rejection.
If autoimmunity to hsp60 plays a role in allograft rejection, modulation of hsp60 autoimmunity might modify the rejection process. We previously have found that hsp60 autoimmunity can be modulated in wild-type NOD (1–5) and other mice (23) by therapeutically vaccinating the mice s.c. with mouse hsp60 peptides or with full-length recombinant hsp60 emulsified in mineral oil (incomplete Freund’s adjuvant, IFA). The mechanism of hsp60 modulation appears to involve a shift in the phenotype of the T cell response specifically to self hsp60 from a Th1 type of response to a Th2 type of response (18). As Th1 type responses have been reported to be involved in allograft rejection (24), it seemed plausible that such modulation of hsp60 autoimmunity might delay or abrogate the process of rejection of foreign tissues.
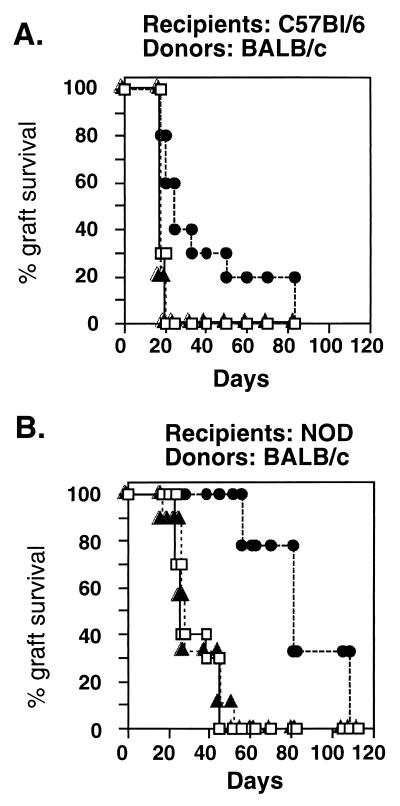
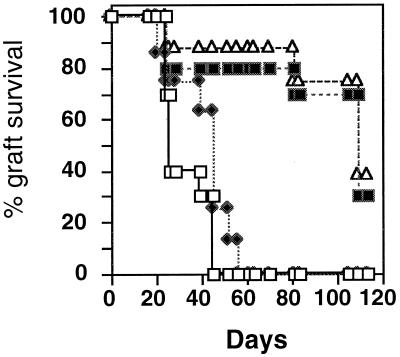
Fig. 4 shows the results of vaccinating wild-type NOD (H-2g7) mice or C57BL/6 (H-2b) mice with full-length recombinant hsp60 2 weeks before transplantation of BALB/c (H-2d) skin. Fig. 4A shows that C57BL/6 mice, a strain not known to suffer from spontaneous autoimmune diseases, manifested prolonged survival of fully allogeneic BALB/c skin grafts after vaccination with hsp60 (P < 0.05). C57BL/6 mice that had been vaccinated with IFA alone or with the control recombinant antigen glutathione transferase did not manifest prolonged survival of the BALB/c skin. It can be seen in Fig. 4B that NOD mice, too, manifested delayed rejection of partially allogeneic BALB/c skin after hsp60 vaccination, compared with vaccination with the control recombinant antigen (P < 0.0001). Fig. 5 shows that vaccinating NOD mice with mouse hsp60 peptides also prolonged the survival of the partially allogeneic BALB/c skin (P < 0.0001). It can be seen that treatment with hsp60 peptides p12 or p277 was effective compared with no treatment or to treatment with the control peptide p11, which is not immunogenic in NOD mice (17). Treatment of NOD mice with the MT-p278 peptide of Mycobacterial hsp60 also did not lead to prolonged survival of the allograft (not shown). Thus, vaccination with full-length hsp60 or with mouse hsp60 epitope peptides, shown previously to modulate spontaneous hsp60 autoimmunity (1–4, 17, 18, 23), can significantly inhibit the rejection of foreign skin, both in NOD and C57BL/6 mice.
Figure 4.
Treatment with hsp60 induces delayed rejection of skin allografts. C57BL/6 (H-2b) mice (A) or wild-type NOD (H-2g7) mice (B) were treated with a single dose of recombinant full-length hsp60 (50 μg in IFA; Sigma) administered s.c. into the back 2 weeks before transplantation of BALB/c (H-2d) skin. Control mice were untreated (□) or treated with IFA containing 50 μg of the recombinant control glutathione transferase (▵). The differences in rejection between treatment with hsp60 (●) and the controls, determined by using the Wilcoxon test, were significant (P < 0.05 for A and P < 0.0001 for B). Each group contained 10–12 mice. The experiment was repeated three times with similar results.
Figure 5.
Treatment with hsp60 peptides induces delayed rejection of skin allografts. Wild-type NOD (H-2g7) mice were treated with various hsp60 peptides (50 μg) in IFA. Control mice were untreated (□) or were treated with IFA containing peptide p11, which is nonimmunogenic in NOD mice (♦) (26). The differences in rejection between treatment with hsp60 epitope peptides p12 (■) or p277 (▵) and the controls were significant (P < 0.0001). The experiment was repeated three times with similar results.
DISCUSSION
The hsp60 molecule of the various mouse strains described in the above experiments is identical in sequence. How might hsp60 expression and autoimmunity to the hsp60 molecule influence the allograft rejection, an immune reaction triggered by foreign transplantation antigens? As we show here, in the process of allograft rejection the expression of hsp60 is up-regulated in the rejected skin. This augmented expression of hsp60 enhances the rejection process. Healthy mice and humans are populated with T cells reactive to self hsp60 (12, 13). Indeed, Moliterno et al. (25) have shown that T cells reactive to hsp60 are present in cardiac allografts. The present paper demonstrates that mice deficient in their spontaneous T cell autoimmunity to hsp60 are slower in rejecting allografts. Moreover, shifting the autoimmune reactivity to hsp60 from a Th1 to a Th2 mode by hsp60 peptide vaccination delays allograft rejection.
Based on these data, we suggest the following model: the process of allograft rejection, initiated by an immune reaction targeted at foreign antigens, causes a local augmentation in the expression of hsp60. The T cells targeted at self hsp60, which normally exist in healthy individuals, reach the site, proliferate locally, and, in conjunction with their enhanced activation, promote the destructive process, culminating in the “final hit.” Thus the tissue specificity of the rejection process is determined by the original insult, the immune reactivity to the alloantigen. Only after the tissue-specific immune process has begun and has caused local damage does autoimmunity to the ubiquitous (and now locally overexpressed) hsp60 enter the scene, acting to augment and complete the destruction. Besides any direct effect in augmenting the rejection process, these anti-hsp60 T cells also could enhance the local immune response to foreign antigens by secreting Th1-type cytokines, such as IFN-γ (18, 26). Conversely, hsp60 autoimmune T cells that have been induced by vaccination to switch from production of IFN-γ to production of Th2 cytokines, such as IL-10 (18) could serve to shut down the effector immune reaction by a form of bystander suppression (27). This working hypothesis has to be established at the molecular level. Preliminary studies, however, indicate that mice treated with hsp60 peptides before receiving skin allografts manifest a 2-fold increase of IL-10 production and a 2-fold decrease in IFN-γ production upon activation in vitro by culture with allogeneic stimulator cells (not shown).
The present results demonstrate that enhanced expression of hsp60 and naturally occurring immunity to self hsp60 can modulate the response to foreign antigens, strong or weak. Hsp60 autoimmunity is not a mere aberration, but has a functional role in enhancing the rejection of foreign tissues. Furthermore, we demonstrate that hsp60 plays a pivotal role in signaling “danger” (28) and enhancing the immune response to foreign antigens. This finding might explain the presence of the hsp60 molecule in the limited set of dominant self-antigens to which there is natural autoimmunity, the immunological homunculus (29, 30). Beyond theoretical considerations, manipulating hsp60 autoimmunity may be therapeutically useful, as we show here, in attenuating graft rejection.
Acknowledgments
Parts of this study were supported by Peptor Ltd. and the Tauro Foundation. O.S.B. is a recipient of the Foukles Foundation Scholarship through the Israel Academy of Sciences. I.R.C. is the incumbent of the Mauerberger Chair of Immunology and the Director of the Robert Koch-Minerva Center for Research in Autoimmune Diseases.
ABBREVIATIONS
- hsp60
60-kDa heat shock protein
- IFA
incomplete Freund’s adjuvant
References
- 1.Elias D, Markovits D, Reshef T, van der Zee R, Cohen I R. Proc Natl Acad Sci USA. 1990;87:1576–1580. doi: 10.1073/pnas.87.4.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elias D, Reshef T, Birk O S, van der Zee R, Walker M D, Cohen I R. Proc Natl Acad Sci USA. 1991;88:3088–3091. doi: 10.1073/pnas.88.8.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elias D, Cohen I R. Lancet. 1994;343:704–706. doi: 10.1016/s0140-6736(94)91582-2. [DOI] [PubMed] [Google Scholar]
- 4.Elias D, Cohen I R. Diabetes. 1995;44:1132–1138. doi: 10.2337/diab.44.9.1132. [DOI] [PubMed] [Google Scholar]
- 5.Birk O S, Elias D, Weiss A S, Rosen A, van-der-Zee R, Walker M D, Cohen I R. J Autoimmun. 1996;9:159–166. doi: 10.1006/jaut.1996.0019. [DOI] [PubMed] [Google Scholar]
- 6.Birk O S, Cohen I R. Curr Opin Immunol. 1993;5:903–909. doi: 10.1016/0952-7915(93)90104-z. [DOI] [PubMed] [Google Scholar]
- 7.Cohen I R. Annu Rev Immunol. 1991;9:567–589. doi: 10.1146/annurev.iy.09.040191.003031. [DOI] [PubMed] [Google Scholar]
- 8.Res P, Thole J, de Vries R. Springer Semin Immunopathol. 1991;13:81–98. doi: 10.1007/BF01225280. [DOI] [PubMed] [Google Scholar]
- 9.van Eden W, de Vries R R. Br J Rheumatol. 1989;28, Suppl. 1:51–53. doi: 10.1093/rheumatology/xxviii.suppl_1.51. [DOI] [PubMed] [Google Scholar]
- 10.Selmaj K, Brosnan C F, Raine C S. Proc Natl Acad Sci USA. 1991;88:6452–6456. doi: 10.1073/pnas.88.15.6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mor F, Cohen I R. J Clin Invest. 1992;90:2447–2455. doi: 10.1172/JCI116136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaufmann S H E. Immunol Today. 1990;11:129–136. doi: 10.1016/0167-5699(90)90050-j. [DOI] [PubMed] [Google Scholar]
- 13.Cohen I R. In: Stress Proteins in Medicine. van Eden W, Young D B, editors. New York: Dekker; 1996. pp. 93–102. [Google Scholar]
- 14.Young R A, Elliott T J. Cell. 1989;59:5–8. doi: 10.1016/0092-8674(89)90861-1. [DOI] [PubMed] [Google Scholar]
- 15.Towbin H, Staehelin T, Gordon J. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boog C J, de Graeff-Meeder E R, Lucassen M A, van der Zee R, Voorhorst-Ogink M M, van Kooten P J, Geuze H J, van Eden W. J Exp Med. 1992;175:1805–1810. doi: 10.1084/jem.175.6.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bockova J, Elias D, Cohen I R. J Autoimmun. 1997;10:323–329. doi: 10.1006/jaut.1997.0150. [DOI] [PubMed] [Google Scholar]
- 18.Elias D, Meilin A, Ablamunits V, Birk O S, Carmi P, Konen-Waisman S, Cohen I R. Diabetes. 1997;46:758–764. doi: 10.2337/diab.46.5.758. [DOI] [PubMed] [Google Scholar]
- 19.Birk O S, Douek D C, Elias D, Takacs K, Dewchand H, Gur S L, Walker M D, van der Zee R, Cohen I R, Altmann D M. Proc Natl Acad Sci USA. 1996;93:1032–1037. doi: 10.1073/pnas.93.3.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kampgen E, Koch N, Koch F, Stoger P, Heufler C, Schuler G, Romani N. Proc Natl Acad Sci USA. 1991;88:3014–3018. doi: 10.1073/pnas.88.8.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott D M, Ehrmann I E, Ellis P S, Bishop C E, Agulnik A I, Simpson E, Mitchell M J. Nature (London) 1995;376:695–698. doi: 10.1038/376695a0. [DOI] [PubMed] [Google Scholar]
- 22.Venner T J, Gupta R S. Biochim Biophys Acta. 1990;1087:336–338. doi: 10.1016/0167-4781(90)90008-p. [DOI] [PubMed] [Google Scholar]
- 23.Elias D, Cohen I R. Diabetes. 1996;45:1168–1172. doi: 10.2337/diab.45.9.1168. [DOI] [PubMed] [Google Scholar]
- 24.Romagniani S. Clin Immunol Immunopathol. 1996;80:225–235. doi: 10.1006/clin.1996.0118. [DOI] [PubMed] [Google Scholar]
- 25.Moliterno R, Aldivia L, Pan F, Duquesnoy R J. Transplantation. 1995;59:598–604. [PubMed] [Google Scholar]
- 26.Finkelman F D, Holmes J, Katona I M, Urban J F, Beckmann M P, Park L S, Schooley K A, Coffman R L, Mosmann T R, Paul W E. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 27.Weiner H L, Friedman A, Miller A, Khoury S J, al-Sabbagh A, Santos L, Sayegh M, Nussenblatt R B, Trenthama D E, Hafler D A. Annu Rev Immunol. 1994;12:809–837. doi: 10.1146/annurev.iy.12.040194.004113. [DOI] [PubMed] [Google Scholar]
- 28.Matzinger P. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 29.Cohen I R, Young D. Immunol Today. 1991;12:105–110. doi: 10.1016/0167-5699(91)90093-9. [DOI] [PubMed] [Google Scholar]
- 30.Cohen I R. Immunol Today. 1992;13:490–494. doi: 10.1016/0167-5699(92)90024-2. [DOI] [PubMed] [Google Scholar]