Abstract
Antiphospholipid syndrome (APS) is characterized by recurrent fetal loss, repeated thromboembolic phenomena, and thrombocytopenia. The syndrome is believed to be caused by antiphospholipid beta-2-glycoprotein-I (β2GPI)-dependent Abs or anti-β2GPI Abs by themselves. Using a hexapeptide phage display library, we identified three hexapeptides that react specifically with the anti-β2GPI mAbs ILA-1, ILA-3, and H-3, which cause endothelial cell activation and induce experimental APS. To enhance the binding of the peptides to the corresponding mAbs, the peptides were lengthened to correspond with the site of the β2GPI epitope being recognized by these mAbs. As a result, the following three peptides were prepared: A, NTLKTPRVGGC, which binds to ILA-1 mAb; B, KDKATFGCHDGC, which binds to ILA-3 mAb; and C, CATLRVYKGG, which binds to H-3 mAb. Peptides A, B, and C specifically inhibit both in vitro and in vivo the biological functions of the corresponding anti-β2GPI mAbs. Exposure of endothelial cells to anti-β2GPI mAbs and their corresponding peptides led to the inhibition of endothelial cell activation, as shown by decreased expression of adhesion molecules (E-selectin, ICAM-1, VCAM-1) and monocyte adhesion. In vivo infusion of each of the anti-β2GPI mAbs into BALB/c mice, followed by administration of the corresponding specific peptides, prevented the peptide-treated mice from developing experimental APS. The use of synthetic peptides that focus on neutralization of pathogenic anti-β2GPI Abs represents a possible new therapeutic approach to APS.
Keywords: anti-β2glycoprotein-I, peptide phage display library, anticardiolipin
Antiphospholipid syndrome (APS), also known as Hughes’ syndrome, is characterized by the presence of high titers of anticardiolipin-β-2-glycoprotein-I (β2GPI)-dependent Abs and/or lupus anticoagulant associated with thromboembolic phenomena, thrombocytopenia, and recurrent fetal loss. Its clinical presentation also includes heart involvement (Libman–Sacks endocarditis). APS can emerge as a primary or secondary effect of systemic lupus erythematosus and other diseases (1–4).
Anticardiolipin-β2GPI-dependent polyclonal Abs bind anionic phospholipids through the β2GPI molecule (2, 5–8). β2GPI (50 kDa), the target antigen for many of the autoimmune anti-β2GPI Abs, is composed of five consecutive consensus repeats (9, 10). β2GPI exhibits several properties in vitro, which define it as an anticoagulant (e.g., inhibition of prothrombinase activity, ADP-induced platelet aggregation, platelet factor IX production) (10). It binds negatively charged phospholipids through a lysine-rich locus (Cys281–Cys288) located in the fifth domain (11). The latter was proposed to contain the target epitope for binding anti-β2GPI Abs as well (12).
It has been postulated that anti-β2GPI Abs exert a direct pathogenic effect by interfering with homeostatic reactions occurring on the surface of platelets or vascular endothelial cells (ECs) (13–15). Passive transfer of these Abs into naive mice resulted in the induction of experimental APS (16). Recently, we (17) and others (15, 18) demonstrated that human polyclonal and monoclonal β2GPI Abs react with ECs and induce differential EC activation in vitro.
In the current study, we describe the ability of peptides derived from a peptide phage display library to prevent experimental APS and neutralize the functional activity of anti-β2GPI mAbs in vitro.
MATERIALS AND METHODS
Origin of the Anti-β2GPI mAbs.
The human anti-β2GPI mAbs designated ILA-1 and ILA-3 (IgMs) were prepared by the human–human hybridoma technique from a patient with APS (17). The H-3 mAb was prepared as previously described (19). All three anti-β2GPI mAbs were found to activate ECs in vitro and to induce experimental APS by passive transfer (17, 19).
Identification of Peptides That Bind Specifically to the Anti-β2GPI mAbs.
The hexapeptide phage display library, kindly provided by George P. Smith (University of Missouri, Columbia, MO), was constructed by use of the phage fd-derived vector fUSE5 as described (20). This library consists of 2 × 108 original phage clones, each containing a hexapeptide fused to the minor coat protein PIII (20). A library sample containing 3.8 × 109 infectious phage particles was subjected to four rounds of selection (panning) and amplification, as previously described (21). Thereafter, individual bacterial colonies containing amplified phage clones were grown in microtiter plates overnight at 37°C, and the phage were tested by ELISA for their ability to specifically bind the mAb. Positive phage clones were propagated and their DNA was sequenced in the epitope region, by using the FSTAQ method with big deoxy termination using an ABI 377 instrument and the fUSE sequencing primer according to the manufacturer’s instructions (Perkin–Elmer).
Synthetic Peptides Used in This Study.
We identified three hexapeptides from the library. Peptide LKTPRV binds specifically to ILA-1 mAb (IC5010−4 M), peptide KDKATF binds specifically to ILA-3 mAb (IC5010−6 M), and peptide TLRVYK binds specifically to H-3 mAb (IC5010−5 M). Because the binding of all three peptides to their specific mAbs showed low affinity, they were lengthened by four to six amino acid residues. Among the elongated peptides thus synthesized, the following were found suitable for carrying out the experiments described below: A, NTLKTPRVGGC binds specifically to ILA-1 mAb (IC5010−8 M); B, KDKATFGCHDGC binds specifically to ILA-3 mAb (IC5010−9 M); C, CATLRVYKGG binds specifically to H-3 mAb (IC5010−7 M); and D, PVRSPHQSYC was used as a control.
The peptides were prepared by conventional solid-phase peptide synthesis, by using an Abimed AMS-422 automated solid-phase multiple peptide synthesizer (Abimed Analysentechnik, Langenfeld, Germany). For purity determination, analytical reversed-phase HPLC was performed by using a prepacked Lichrosphere-100 RP-18 column (Merck).
Preparation of Affinity Peptide Column.
Peptide affinity columns were prepared via addition of the thiol-containing peptide to MBPH [4-(4N-maleimidophenyl)-butyric acid hydrazide-HCl] (Pierce no. 2234) and coupling the peptide adduct with CNBr-activated Sepharose-4B (Amersham Pharmacia no. 17–0430–01), according to the manufacturer’s instructions. The antibody fractions adsorbed specifically by the different affinity chromatography columns were eluted with HCl–glycine buffer (0.1M, pH 2.2).
Peptide Biotinylation.
Eleven mg of resin-bound peptides (Wang Resin, Calbiochem–Nova Biochem) were suspended in N-methyl-2-pyrrolidone, and 15 mmol of biotin-N-hydroxysuccinimide (Sigma), and 15 mmol of di-isopropylethylamine were added to the peptide mixture. After 16 hr, the biotinylated peptides were deprotected and cleaved from the resin by a cleavage mixture containing 5% triethylsilan (Fluka), 5% water, and 90% trifluoroacetic acid. The cleaved peptides were precipitated with ice-cold peroxide-free ether, and the pellet was dissolved in water and subsequently lyophilized. Biotinylated peptides were purified by HPLC by using 0.1% trifluoroacetic acid in 20% H2O in acetonitrile.
Inhibition of Binding of Anti-β2GPI mAbs to β2GPI by the Different Peptides.
Binding of anti-β2GPI Ab to β2GPI in the presence of the different peptides was performed on γ-irradiated ELISA plates coated with β2GPI (10 μg/ml) and blocked with 1% gelatin. The anti-β2GPI mAbs ILA-1, ILA-3, and H-3, at 50% binding (corresponding to 5, 3.5, and 5 μg/ml respectively), were incubated with varying concentrations of the peptides A, B, and C, respectively. The reaction mixture was then transferred to the β2GPI-coated plates and assayed as described (17, 19). The percentage of inhibition was calculated as follows: % inhibition = [OD anti-β2GPI–OD anti- β2GPI with inhibitor]/OD anti-β2GPI ×100.
Inhibition of Binding of Anti-β2GPI mAbs to ECs in the Presence of the Different Peptides.
Human umbilical vein ECs (HUVEC) were isolated as previously described and cultured under standard conditions (22). Cells were used at passages 1 or 2 and plated onto gelatin-coated 96-well plates. Cyto-ELISA was performed as described (15). The inhibition of the anti-β2GPI mAbs binding to the ECs by the different peptides was carried out analogously to the procedure described in the previous section.
Binding of Anti-β2GPI Abs to the Studied Peptides.
Streptavidin-coated plates were incubated with biotinylated peptides and blocked with 3% BSA. Affinity-purified human anti-β2GPI Abs, from sera of patients with APS, were added at different concentrations and probed with antihuman-IgM/IgG conjugated to alkaline phosphatase followed by the addition of appropriate substrate.
Adherence of U937 Monocyte Cells to ECs in the Presence of Anti-β2GPI mAbs and the Specific Peptides.
This assay was performed as previously described (23) and modified by us (17). Adhesion assays were performed on HUVEC monolayers that had been preincubated sequentially with β2GPI and with various anti-β2GPI mAbs and different concentrations of specific or control peptides. The results are expressed as a percentage of added adhered thymidine-labeled U937 cells and are presented as the mean ± SD from three replicate wells.
Detection of the Adhesion Molecule Expression on ECs after Exposure to Anti-β2GPI mAbs and the Different Peptides.
The detection of adhesion molecules on ECs was performed by ELISA as previously described (24). Fixed HUVEC cells were preincubated sequentially with β2GPI and anti-β2GPI mAbs with and without different concentrations of specific and irrelevant peptides. The HUVEC cells were treated with PBS containing 0.2% Triton X-100 to permeabilize the cell membrane (24). After blocking with 3% BSA, 1 μg/ml of biotinylated mouse antihuman E-selectin, antihuman intracellular adhesion molecule-1 (ICAM-1) or antihuman vascular cell adhesion molecule-1 (VCAM-1) (PharMingen), were added for 1 hr (21). The cells were then exposed to streptavidin alkaline phosphatase (Jackson ImmunoResearch) and an appropriate substrate.
Prevention of the Induction of Experimental APS in Mice.
Pregnant BALB/c mice were infused intravenously with 20 μg of each of the three mAbs (ILA-1, ILA-3, or H-3) at day 0 of pregnancy (the day on which a vaginal plug was observed after mating) (16, 17). Eight hours later, each mouse received an intravenous infusion of 40 μg per mouse of the tested peptides A, B, C, or D. The mice were sacrificed on day 15 of pregnancy. Fetal resorptions, activated partial thromboplastin times, and platelet counts (markers of the APS equivalent in mice) were determined as previously described (16, 17).
Statistical Analysis.
Differences between the binding properties and biological activities of the different groups were evaluated by ANOVA. Values of P < 0.05 were considered as statistically significant.
RESULTS
Isolation of phage displaying peptides recognized by anti-β2GPI monoclonal Abs.
The hexapeptides displayed on phage and recognized by biotinylated ILA-1, ILA-3, or H-3 mAbs were determined. ILA-1 mAb detected a peptide with the sequence LKTPRV, which appeared in 24 clones and is a mimotope representing domains I and II, closely resembling the sequence LK(C)TPRV on the native form of the β2GPI molecule (amino acids 58–63). The other 18 clones showed the motif KTPRVT, a mimotope that is found at the above location on the β2GPI and appears as K(C)TPRV(CC)T. ILA-3 mAb recognized a peptide with the linear sequence KDKATF and is located in the fourth domain of the β2GPI molecule (amino acids 208–213), identified in 15 clones. The anti- β2GPI mAb H-3 recognized a linear motif sequence TLRVYK, located in the third domain of the β2GPI molecule (amino acids 133–138) and identified in 37 clones.
Inhibition of Binding of Anti-β2GPI mAbs to β2GPI and ECs by the Synthetic Peptides A, B, and C.
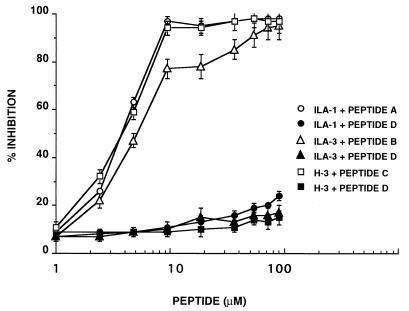
The specificity of interaction of each of the mAbs with the corresponding synthetic peptides A, B, and C (see Materials and Methods) was assessed by inhibition experiments, in which the binding of the anti-β2GPI mAbs to β2GPI was inhibited by the peptides. As shown in Fig. 1, the synthetic peptide A (10 μM) inhibited the binding of ILA-1 mAb to β2GPI by 97% under the experimental conditions used, whereas peptide D (the control peptide) at the same concentration showed only a minor inhibitory effect (8%). Peptide B (10 μM) inhibited the binding of ILA-3 mAb to β2GPI by 77%. Binding of H-3 mAb to β2GPI was inhibited by 98% by peptide C (10 μM).
Figure 1.
Inhibition of the binding of anti-β2GPI mAbs (ILA-1, ILA-3, and H-3) to β2GPI by peptides A, B, and C, respectively, was performed as described in Materials and Methods. Peptide D was used as control. Each point represents the mean ± SD of three separate experiments.
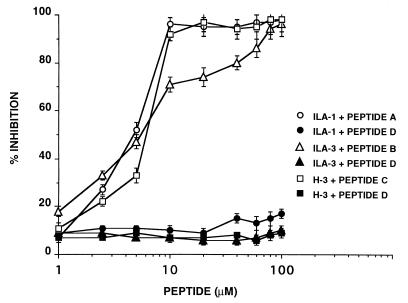
The specific inhibitory effects of the individual peptides on the binding of anti- β2GPI mAbs to HUVEC are documented in Fig. 2. Under the experimental conditions used, peptides A, B, and C, each at 10 μM, inhibited the specific binding of mAbs ILA-1, ILA-3, and H-3 by 96%, 71%, and 92%, respectively. No inhibition of binding of the anti-β2GPI mAbs to HUVEC by peptide D was observed (P > 0.05).
Figure 2.
Inhibition of the binding of anti-β2GPI mAbs (ILA-1, ILA-3, and H-3) to HUVEC by peptides A, B, and C, respectively, was carried out as described in Materials and Methods. Peptide D was used as control. Each point represents the mean ± SD of three separate experiments.
Prevention of Monocyte Adhesion to HUVEC after Specific Neutralization of Anti-β2GPI mAbs by the Synthetic Peptides A, B, and C.
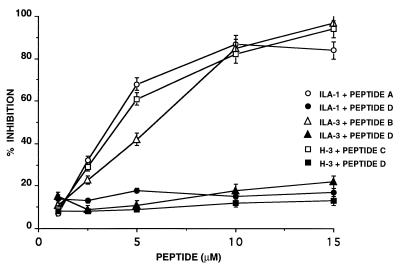
The adhesion of monocytes to ECs on their exposure to anti-β2GPI mAbs serves as a marker of EC activation. We examined the ability of the peptides to prevent monocyte adhesion to HUVEC after neutralization of the anti-β2GPI mAbs with their corresponding specific synthetic peptides (Fig. 3). ILA-1 mAb, after preincubation with peptide A, inhibited the adhesion of U937 to ECs by 84% under the experimental conditions used, compared with the inhibition of 6–9% seen in the presence of the control peptide D. The most significant inhibition was observed after preincubation of ILA-3 with peptide B and of H-3 mAb with peptide C (Fig. 3). In the former case, inhibition of adhesion of up to 97% was recorded, and in the latter case it reached 94%. In both cases the peptide concentration was 15 μM. No significant inhibition of adhesion of U937 to ECs was recorded in the presence of the anti-β2GPI mAbs and peptide D (P > 0.05).
Figure 3.
Inhibition by peptide A, B, or C of monocyte adhesion to HUVEC after incubation with anti-β2GPI mAbs ILA-1, ILA-3, or H-3. Peptide D was used as control. Each point represents the mean ± SD of three separate experiments.
Prevention of the Expression of Adhesion Molecules on HUVEC on Exposure to Anti-β2GPI mAbs Preincubated with the Synthetic Peptides.
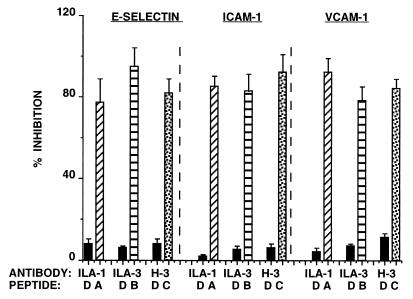
As a result of exposure of the ECs sequentially to β2GPI and anti-β2GPI mAbs, the increase in adhesion of monocytes to activated ECs was followed by an increase in the expression of adhesion molecules (17). We examined the effects of the specific peptides on the expression of adhesion molecules on HUVEC on exposure of the cells to anti-β2GPI mAbs. The data presented in Fig. 4 show clearly that the effects of the anti-β2GPI mAbs on E-selectin, ICAM-1, and VCAM-1 expression were markedly inhibited by their corresponding peptides (P < 0.001 for each of the specific peptides, in comparison to peptide D).
Figure 4.
Inhibition by peptides A, B, and C of the expression of E-selectin, ICAM-1, and VCAM-1 on HUVEC preincubated with the anti-β2GPI mAbs ILA-1 (diagonal ladder column), ILA-3 (ladder column), and H-3 (spotted column), respectively. Peptide D (solid column) was used as control. Each point represents the mean ± SD of three separate experiments.
Prevention of Induction of Experimental APS in Mice by Peptides A, B, and C.
Increased fetal loss, thrombocytopenia, and prolonged activated partial thromboplastin time (aPTT) were induced in naive mice after passive intravenous administration of the three pathogenic anti-β2GPI mAbs ILA-1, ILA-3, or H-3 (Table 1). The clinical manifestations of experimental APS could be prevented by subsequent infusion of the corresponding specific peptides.
Table 1.
Clinical manifestations in mice infused with anti-β2GP1 mAbs and thereafter with peptides A, B, or C
| Infusion of mAb | mAb ILA-1
|
mAb ILA-3
|
mAb H-3
|
|||
|---|---|---|---|---|---|---|
| Peptide A | Peptide D | Peptide B | Peptide D | Peptide C | Peptide D | |
| aPTT, sec* | 33 ± 4 | 72 ± 5 | 27 ± 2 | 64 ± 3 | 31 ± 4 | 83 ± 5 |
| (P < 0.02)‡ | (P < 0.02)‡ | (P < 0.02)‡ | ||||
| Platelet count, | 989 ± 103 | 498 ± 142 | 1137 ± 219 | 579 ± 163 | 1,242 ± 267 | 499 ± 12 |
| cells/mm3 × 10−3 | (P < 0.003)‡ | (P < 0.002)‡ | (P < 0.001)‡ | |||
| % fetal loss† | 8 ± 2 | 39 ± 3 | 6 ± 2 | 42 ± 3 | 7 ± 2 | 42 ± 4 |
| (P < 0.001)‡ | (P < 0.001)‡ | (P < 0.001)‡ | ||||
Values are expressed as means ± SD of two experiments: N = 11–15 mice in each group.
aPTT, activated partial thromboplastin time.
Percent of fetal loss = resorbed fetuses/total fetuses.
Statistical analyses were performed by ANOVA. No statistical significance (P > 0.05) in the clinical manifestation, as noticed in mice treated with the control peptide D, compared to nontreated mice.
The data presented in Table 1 show that BALB/c mice that were preinfused with anti-β2GPI mAb ILA-1 and then treated with peptide A showed normal values of fetal loss (8 ± 2%) compared with mice with experimental APS (39 ± 3%) (P < 0.001), under the experimental conditions specified in Materials and Methods. No decrease in platelet count was noted in these treated mice when compared with APS mice (P < 0.003). Activated partial thromboplastin time in mice that received ILA-1 mAb and were treated with peptide A (33 ± 4 sec) was not prolonged, whereas significant prolongation (72 ± 5 sec) was seen in mice with experimental APS (P < 0.02).
The same pattern of symptom prevention by specific peptides was detected in mice that received pathogenic anti-β2GPI ILA-3 and were treated with peptide B or that received H-3 mAb and were treated with peptide C (Table 1).
Detection and Quantitative Estimation of the Fractions of Human Anti-β2GPI Abs Specifically Recognizing Peptides A, B, or C in APS Patients.
Ten of 43 APS patients were found to contain Abs to one, two, or all three peptides described above. Polyclonal affinity-purified anti-β2GPI Abs from 10 APS patients were sequentially loaded on chromatography columns containing immobilized peptides A, B, or C. Three fractions obtained from each patient were capable of binding specifically to peptide A, B, or C, as ascertained by ELISA by using biotinylated peptides bound to streptavidin plates. Amounts of the three anti-β2GPI specific fractions, expressed as percentage of the total anti-β2GPI in these patients, are recorded in Table 2. The percentages of the corresponding fractions in these patients are also shown in Table 2. The percentage of anti-β2GPI Abs that recognized peptides A, B, or C from the total anti-β2GPI Abs, varied between 0.4% and 43%.
Table 2.
Fractions of human anti-β2GP1 Abs recognizing peptide A, B, or C in patients with APS
| Patient no. | Anti-peptide A
|
Anti-peptide B
|
Anti-Peptide C
|
Total anti-β2GP1 in μg/ml serum | |||
|---|---|---|---|---|---|---|---|
| μg/ml* | %† | μg/ml* | %† | μg/ml* | %† | ||
| 1 | 87.6 | 23 | 140.9 | 37 | 1.5 | 0.4 | 381 |
| 2 | 1.6 | 0.7 | 34.3 | 15 | 6.9 | 3 | 229 |
| 3 | 4.9 | 1.4 | 1.8 | 90.5 | 135.3 | 39 | 347 |
| 4 | 83.8 | 29 | 124.3 | 43 | 63.6 | 22 | 289 |
| 5 | 1.4 | 0.9 | 44.4 | 29 | 2.7 | 1.8 | 153 |
| 6 | 18.2 | 9.0 | 28.3 | 14 | 8.1 | 3 | 202 |
| 7 | 52.1 | 27 | 21.2 | 11 | 0.9 | 0.5 | 193 |
| 8 | 8.9 | 2.8 | 104.6 | 33 | 13.3 | 4.2 | 317 |
| 9 | 29.5 | 12 | 3.2 | 1.3 | 1.2 | 0.5 | 246 |
| 10 | 1.1 | 0.6 | 13.9 | 7.9 | 29 | 16.4 | 177 |
Affinity-purified anti-β2GP1 Abs were loaded on peptide A column, eluted, and passed through peptide B column followed by peptide C column.
The values given in μg/ml represent IgG plus IgM antibodies as determined by ELISA.
The values given represent percentage of antipeptide antibodies of total anti-β2GPI Abs.
DISCUSSION
Using a hexapeptide phage display library, we identified three hexapeptides that interact specifically with the anti-β2GPI mAbs ILA-1, ILA-3, and H-3, previously shown to exhibit pathogenic activity in vitro and in vivo (17). These peptides correspond to three epitopes located in domains I-II, III, and IV of the β2GPI molecule. To increase the affinity of the three hexapeptides for their Abs, they were lengthened by 4–6 amino acid residues. The elongated peptides were: A, NTLKTPRVGGC; B, KDKATFGTHDGC; and C, CATLRVYKGG. The cysteine residues included within these peptides enabled their coupling with the modified Sepharose carrier (see Materials and Methods), whereas the glycine residues enabled peptide elongation. The finding that elongation of the hexapeptides resulted in higher specificity suggests that whereas short peptides displayed on phage might enable detection of the site recognized on the protein antigen by the specific antibody, peptide elongation might subsequently increase the specific binding.
We have previously shown that ILA-1, ILA-3, and H-3 caused activation of ECs in the presence of β2GPI. The activation was characterized by enhancement of monocyte adhesion to target ECs via elevated expression of adhesion molecules such as ICAM-I, VCAM-I, and E-selectin (17). Infusion of these mAbs into naive mice resulted in induction of experimental APS (17). The data presented in this study show that peptides A, B, and C specifically inhibit the binding of the mAbs to β2GPI as well as to ECs. Prevention of HUVEC activation was accompanied by elimination of the expression of the adhesion molecules ICAM-I, VCAM-I, and E-selectin.
Mice infused with the pathogenic anti-β2GPI mAbs and then treated with the corresponding specific peptides were protected from the induction of experimental APS, as shown by their normal platelet counts and activated partial thromboplastin time values as well as the absence of pregnancy loss.
It was of interest to determine whether human polyclonal anti-β2GPI Abs, affinity-purified from patients with APS by using immobilized β2GPI columns, contain fractions that specifically recognize peptides A, B, and C. Using a sequential fractionation method and columns of immobilized peptides A, B, or C, we were able to demonstrate the existence of different fractions of anti-β2GPI Abs that specifically recognize peptide A, B, or C. The data presented in Table 2 show that in all the patients described (i.e., 10 of 43), the total amounts of the fractions recognizing these three peptides did not account for all of the anti-β2GPI Abs isolated. The percentages of the isolated fractions that specifically recognize peptide A, B, or C ranged between 0.4% and 43%. It is thus obvious that other target epitopes for anti-β2GPI Abs exist in addition to those described in this study. Anti-β2GPI mAbs that differ from ours and recognize an epitope located in the fifth domain of β2GPI (12), in domains I-IV (25), or in domain I (26) have been described. Thus, other anti-β2GPI mAbs derived from APS patients might recognize other epitopes on β2GPI.
The differences in the target epitopes recognized on the β2GPI molecule by different fractions of polyclonal affinity-purified anti-β2GPI Abs might be responsible for the large diversity of the functional activities of anti-β2GPI Abs. The β2GPI molecule itself possesses several in vitro characteristics, such as anticoagulant activity (e.g., inhibition of prothrombinase activity, ADP-induced platelet aggregation, platelet factor IX production, tissue factor release by ECs) (2, 10). The various anti-β2GPI Abs directed to different epitopes of the β2GPI molecule might inactivate the anticoagulant activity of this protein in some patients, thus manifesting a protective function. However, the anti-β2GPI Abs targeting different epitopes on the β2GPI might affect different biological systems. Anti-β2GPI Abs show pathogenic functional biological characteristics on their own: some of them activate ECs (15–18), whereas others may cause thrombus formation in an ex vivo model of thrombosis in the presence of negatively charged phospholipids (27) or induce pregnancy loss when infused into naive mice (16, 17).
The SwissProt protein database revealed high homology between the hexapeptides that bind to ILA-1, ILA-3, and H-3 mAbs and the membrane particles of different bacteria and viruses. The sequence LKTPRV showed homology to eight different bacteria (such as pseudomonas aeroginosa) and homologies to five kinds of viruses including polyoma virus, human cytomegalovirus, and adenovirus. The sequence TLRVYK showed homology to eight different bacteria, including haemophilus influenzae, neisseria gonorrhoae, and shigella dysenteria and to viruses such as EBV and HIV. Our data might therefore support the theory predicting that epitope mimicry is involved in the propagation of the autoimmune status. This theory is based on the assumption that infectious agents (parasites, bacteria, yeast, or viruses) display epitopes that immunologically resemble the host determinants and that the minor antigenic differences between the two enable the pathogen’s epitope to induce an immune response that disrupts tolerance to the host epitope (28–32).
The above findings might suggest a new therapeutic approach for the antiphospholipid syndrome that focuses on neutralization of pathogenic anti-β2GPI Abs. A new sequential fractionation method was developed for isolation of antigen-specific fractions (anti-β2GPI fractions in our case) that specifically recognize the peptides studied. Similar methods might be used in the future to quantify the specific pathogenic fractions in patients with other autoimmune disorders.
Acknowledgments
This study was supported by a grant from the Israel Academy of Sciences and Humanities no. 736/96, Ministry of Science grant no. 5924-3-97, and the Rashi Foundation.
ABBREVIATIONS
- APS
antiphospholipid syndrome
- β2GPI
β-2-glycoprotein-I
- EC
endothelial cell
- HUVEC
human umbilical vein endothelial cells
- mAb
monoclonal antibody
- ICAM-1
intracellular adhesion molecule-1
- VCAM-1
vascular cell adhesion molecule-1
References
- 1.Hughes R V, Harris E N, Gharavi A E. J Rheumatol. 1986;13:486–492. [PubMed] [Google Scholar]
- 2.McNeil H P, Chesterman C N, Krilis S A. Adv Immunol. 1991;49:193–115. doi: 10.1016/s0065-2776(08)60777-4. [DOI] [PubMed] [Google Scholar]
- 3.Hughes G R V. Lancet. 1993;342:341–344. doi: 10.1016/0140-6736(93)91477-4. [DOI] [PubMed] [Google Scholar]
- 4.Tsutsumi A, Matsuura E, Ichikawa K, Fujisaku A, Mukai M, Kobayashi S, Koike T. Arthritis Rheum. 1996;391:466–471. doi: 10.1002/art.1780390905. [DOI] [PubMed] [Google Scholar]
- 5.Galli M, Comfurious P, Massen C, Hemker M H, Be-Bates P J, van-Breda-Vriesman P J. Lancet. 1992;335:1544–1546. doi: 10.1016/0140-6736(90)91374-j. [DOI] [PubMed] [Google Scholar]
- 6.Matsuura E, Igarashi Y, Fujimoto M, Ichikawa K, Suzuki T, Sumida T, Yasuda T, Koike T. J Immunol. 1992;148:3885–3891. [PubMed] [Google Scholar]
- 7.McNeil H P, Simpson R J, Chesterman C N, Krilis S A. Proc Natl Acad Sci USA. 1990;87:4120–4125. doi: 10.1073/pnas.87.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alarcon-Segovia D, Cabral A R. J Rheumatol. 1996;23:8–13. [PubMed] [Google Scholar]
- 9.Schultze H E, Heide H, Haput H. Naturwissenschaften. 1961;48:719–724. [Google Scholar]
- 10.Kandiah D A, Krilis S A. Lupus. 1994;3:207–212. doi: 10.1177/096120339400300401. [DOI] [PubMed] [Google Scholar]
- 11.Hunt J, Krilis S A. J Immunol. 1994;152:653–659. [PubMed] [Google Scholar]
- 12.Wang M X, Kandiah D A, Ichikawa K, Khamashta M, Hughes G, Koike T, Roubey R, Krilis S A. J Immunol. 1995;155:1629–1636. [PubMed] [Google Scholar]
- 13.Shi W, Chong B H, Chesterman C N. Blood. 1993;81:1255–1262. [PubMed] [Google Scholar]
- 14.Pierangeli S S, Liu X W, Anderson G, Barker J H, Harris E N. Circulation. 1996;94:1746–1751. doi: 10.1161/01.cir.94.7.1746. [DOI] [PubMed] [Google Scholar]
- 15.Simantov R, LaSala J M, Lo S K, Gharavi A E, Sammaritano L R, Salmon J E, Silverstein R L. J Clin Invest. 1995;96:2211–2219. doi: 10.1172/JCI118276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blank M, Cohen J, Toder V, Shoenfeld Y. Proc Natl Acad Sci USA. 1991;88:3069–3073. doi: 10.1073/pnas.88.8.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.George J, Blank M, Levy Y, Grinbaum E, Cohen S, Damianovich M, Tincani A, Shoenfeld Y. Circulation. 1998;97:900–905. doi: 10.1161/01.cir.97.9.900. [DOI] [PubMed] [Google Scholar]
- 18.DelPapa N, Guidali L, Sala A, Buccellati C, Khamashta M A, Ichikawa K, Koike T, Balestrieri G, Tincani A, Hughes G R V, et al. Arthritis Rheum. 1997;40:551–561. doi: 10.1002/art.1780400322. [DOI] [PubMed] [Google Scholar]
- 19.Bakimer R, Gilburd B, Zurgil N, Shoenfeld Y. Clin Immunol. Immunopathol. 1993;69:97–102. doi: 10.1006/clin.1993.1155. [DOI] [PubMed] [Google Scholar]
- 20.Scott J K, Smith G P. Science. 1990;249:386–390. doi: 10.1126/science.1696028. [DOI] [PubMed] [Google Scholar]
- 21.Balass M, Heldman J, Cabilly S, Givol D, Katchalski-Katzir E, Fuchs S. Proc Natl Acad Sci USA. 1993;90:10638–10642. doi: 10.1073/pnas.90.22.10638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaffe E A, Nachman R L, Becker C G, Minick C R. J Clin Invest. 1973;5:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carvalho D, Savage C O S, Black C M, Pearson J D. J Clin Invest. 1996;97:111–119. doi: 10.1172/JCI118377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adamson, P., Tighe, M. & Pearson, J. D. (1996) Cell Adhes. Commun.511–525. [DOI] [PubMed]
- 25.Igarashi M, Matsuura E, Igarashi Y, Nagae H, Ichikawa K, Triplett D A, Koike T. Blood. 1996;87:3262–3267. [PubMed] [Google Scholar]
- 26.Iveson G M, Victoria E J, Marquis D M. Proc Natl Acad Sci USA. 1998;95:15542–46. doi: 10.1073/pnas.95.26.15542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olee T, Pierangeli S S, Handley H H, Le DT, Wei X, Lai C J, En J, Novotny W, Harris E, Woods V L, Jr, et al. Proc Natl Acad Sci USA. 1996;93:8606–8611. doi: 10.1073/pnas.93.16.8606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shoenfeld Y, Isenberg D A. Immunol Today. 1998;9:178–181. doi: 10.1016/0167-5699(88)91294-7. [DOI] [PubMed] [Google Scholar]
- 29.Wucherpfennig K W, Strominger J L. Cell. 1995;80:695–705. doi: 10.1016/0092-8674(95)90348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gianani R, Sarvetnic N. Proc Natl Acad Sci USA. 1996;93:2257–2259. doi: 10.1073/pnas.93.6.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davies J M. Immunol Cell Biol. 1997;75:113–126. doi: 10.1038/icb.1997.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oldstone M B. FASEB. 1998;12:1255–1265. doi: 10.1096/fasebj.12.13.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]