Abstract
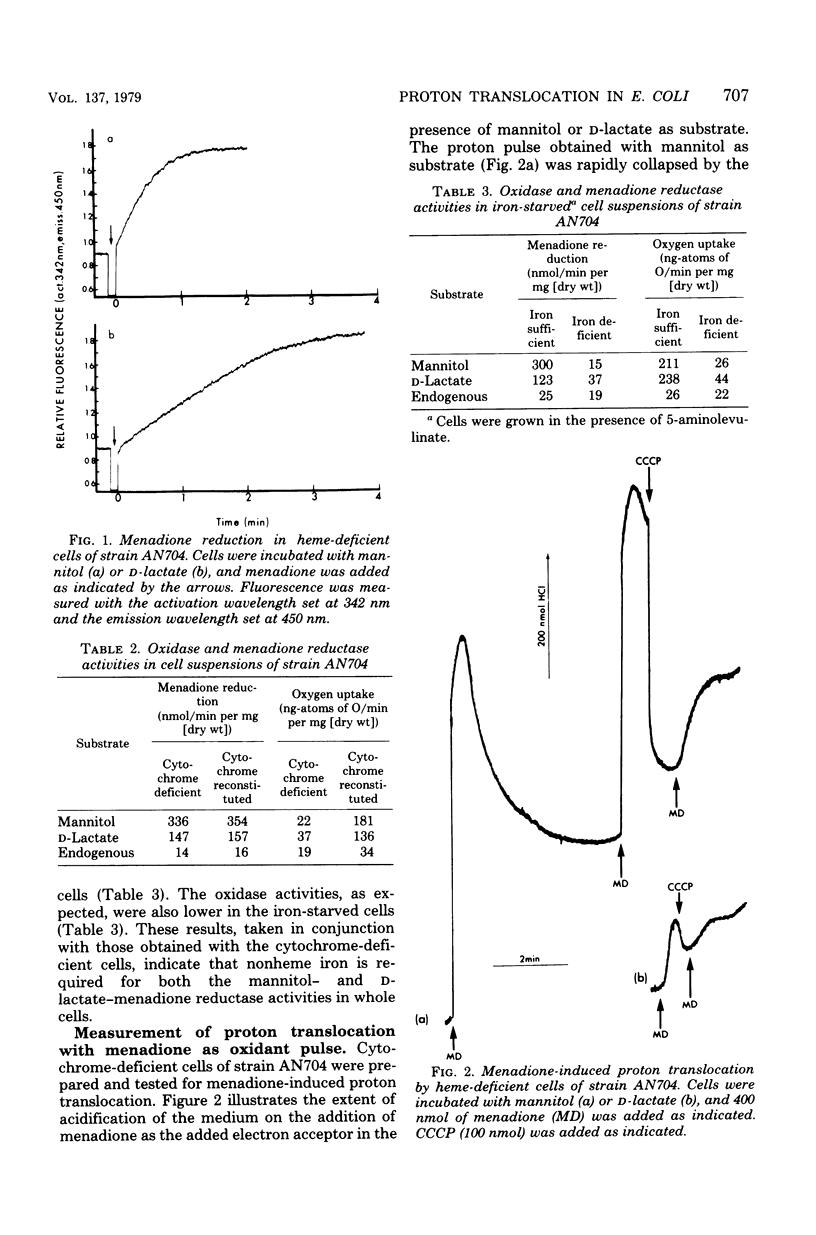
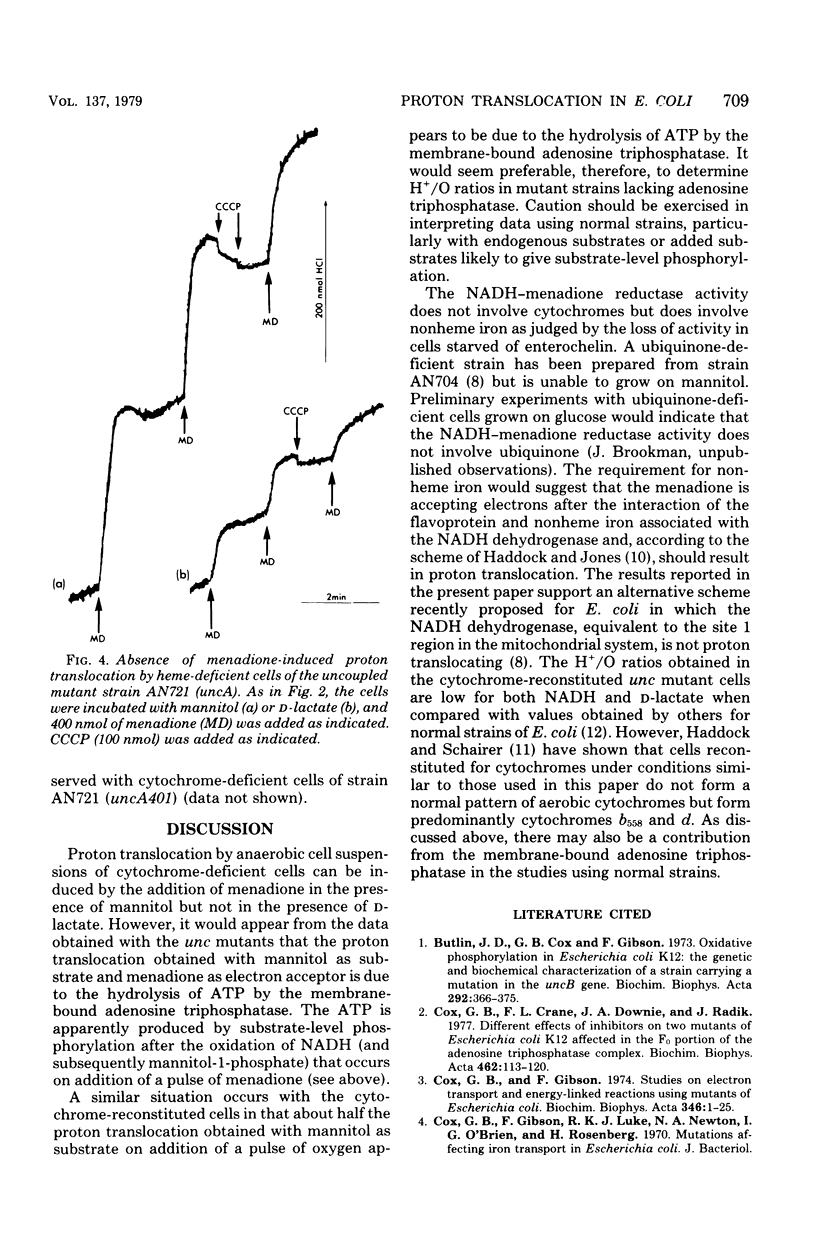
Cytochrome-deficient cells of a strain of Escherichia coli lacking 5-amino-levulinate synthetase have been used to study proton translocation associated with the reduced nicotinamide adenine dinucleotide (NADH) dehydrogenase region of the electron transport chain. Menadione was used as electron acceptor, and mannitol was used as the substrate for the generation of intracellular NADH. The effects of iron deficiency on NADH- and D-lactate-menadione reductase activities were studied in iron-deficient cells of a mutant strain unable to synthesize the iron chelator enterochelin; both activities were reduced. The NADH- menadione reductase activity in cytochrome-deficient cells was associated with proton translocation and could be coupled to the uptake of proline. However proton translocation associated with the NADH-menadione reductase activity was prevented by a mutation in an unc gene. It was concluded that there is no proton translocation associated with the NADH-dehydrogenase region of the electron transport chain in E. coli and that the proton translocation obtained with mannitol as substrate is due to the activity of membrane-bound adenosine triphosphatase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K-12: the genetic and biochemical characterisations of a strain carrying a mutation in the uncB gene. Biochim Biophys Acta. 1973 Feb 22;292(2):366–375. doi: 10.1016/0005-2728(73)90043-1. [DOI] [PubMed] [Google Scholar]
- Cox G. B., Crane F. L., Downie J. A., Radik J. Different effects of inhibitors on two mutants of Escherichia coli K12 affected in the Fo portion of the adenosine triphosphatase complex. Biochim Biophys Acta. 1977 Oct 12;462(1):113–120. doi: 10.1016/0005-2728(77)90193-1. [DOI] [PubMed] [Google Scholar]
- Cox G. B., Gibson F., McCann L. Oxidative phosphorylation in Escherichia coli K12. An uncoupled mutant with altered membrane structure. Biochem J. 1974 Feb;138(2):211–215. doi: 10.1042/bj1380211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Gibson F., McCann L. Reconstitution of oxidative phosphorylation and the adenosine triphosphate-dependent transhydrogenase activity by a combination of membrane fractions from unCA- and uncB- mutant strains of Escherichia coli K12. Biochem J. 1973 Aug;134(4):1015–1021. doi: 10.1042/bj1341015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Gibson F. Studies on electron transport and energy-linked reactions using mutants of Escherichia coli. Biochim Biophys Acta. 1974 Apr 30;346(1):1–25. doi: 10.1016/0304-4173(74)90010-x. [DOI] [PubMed] [Google Scholar]
- Cox G. B., Newton N. A., Gibson F., Snoswell A. M., Hamilton J. A. The function of ubiquinone in Escherichia coli. Biochem J. 1970 Apr;117(3):551–562. doi: 10.1042/bj1170551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie J. A., Cox G. B. Sequence of b cytochromes relative to ubiquinone in the electron transport chain of Escherichia coli. J Bacteriol. 1978 Feb;133(2):477–484. doi: 10.1128/jb.133.2.477-484.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutowski S. J., Rosenberg H. Succinate uptake and related proton movements in Escherichia coli K12. Biochem J. 1975 Dec;152(3):647–654. doi: 10.1042/bj1520647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A., Jones C. W. Bacterial respiration. Bacteriol Rev. 1977 Mar;41(1):47–99. doi: 10.1128/br.41.1.47-99.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A., Schairer H. U. Electron-transport chains of Escherichia coli. Reconstitution of respiration in a 5-aminolaevulinic acid-requiring mutant. Eur J Biochem. 1973 May;35(1):34–45. doi: 10.1111/j.1432-1033.1973.tb02806.x. [DOI] [PubMed] [Google Scholar]
- Lawford H. G., Haddock B. A. Respiration-driven proton translocation in Escherichia coli. Biochem J. 1973 Sep;136(1):217–220. doi: 10.1042/bj1360217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., COHEN-BAZIRE G., COHN M. Sur la biosynthèse de la beta-galactosidase (lactase) chez Escherichia coli; la spécificité de l'induction. Biochim Biophys Acta. 1951 Nov;7(4):585–599. doi: 10.1016/0006-3002(51)90072-8. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Acid-base titration across the membrane system of rat-liver mitochondria. Catalysis by uncouplers. Biochem J. 1967 Aug;104(2):588–600. doi: 10.1042/bj1040588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton N. A., Cox G. B., Gibson F. The function of menaquinone (vitamin K 2 ) in Escherichia coli K-12. Biochim Biophys Acta. 1971 Jul 20;244(1):155–166. doi: 10.1016/0304-4165(71)90132-2. [DOI] [PubMed] [Google Scholar]
- Rosenberg H., Cox G. B., Butlin J. D., Gutowski S. J. Metabolite transport in mutants of Escherichia coli K12 defective in electron transport and coupled phosphorylation. Biochem J. 1975 Feb;146(2):417–423. doi: 10.1042/bj1460417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West I. C., Mitchell P. Stoicheiometry of lactose-H+ symport across the plasma membrane of Escherichia coli. Biochem J. 1973 Mar;132(3):587–592. doi: 10.1042/bj1320587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young I. G., Langman L., Luke R. K., Gibson F. Biosynthesis of the iron-transport compound enterochelin: mutants of Escherichia coli unable to synthesize 2,3-dihydroxybenzoate. J Bacteriol. 1971 Apr;106(1):51–57. doi: 10.1128/jb.106.1.51-57.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young I. G., Wallace B. J. Mutations affecting the reduced nicotinamide adenine dinucleotide dehydrogenase complex of Escherichia coli. Biochim Biophys Acta. 1976 Dec 6;449(3):376–385. doi: 10.1016/0005-2728(76)90149-3. [DOI] [PubMed] [Google Scholar]


