Abstract
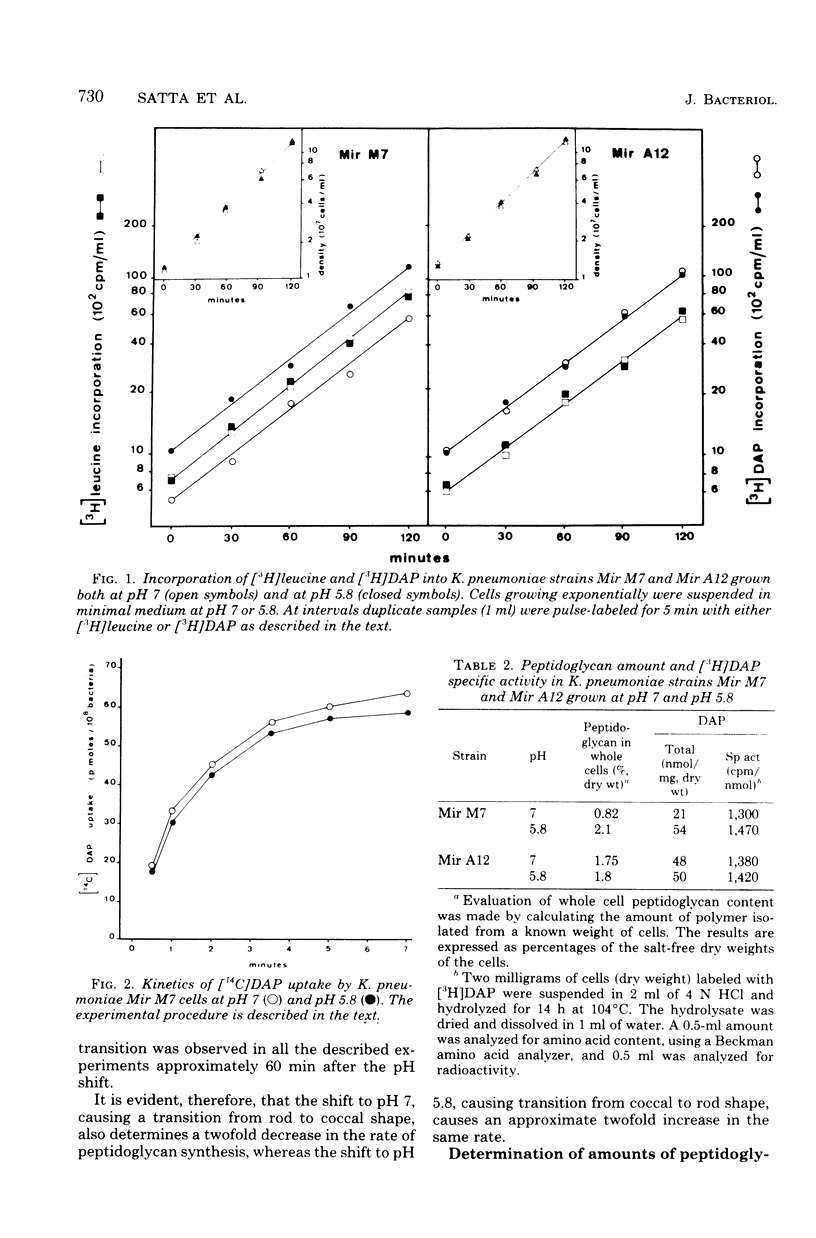
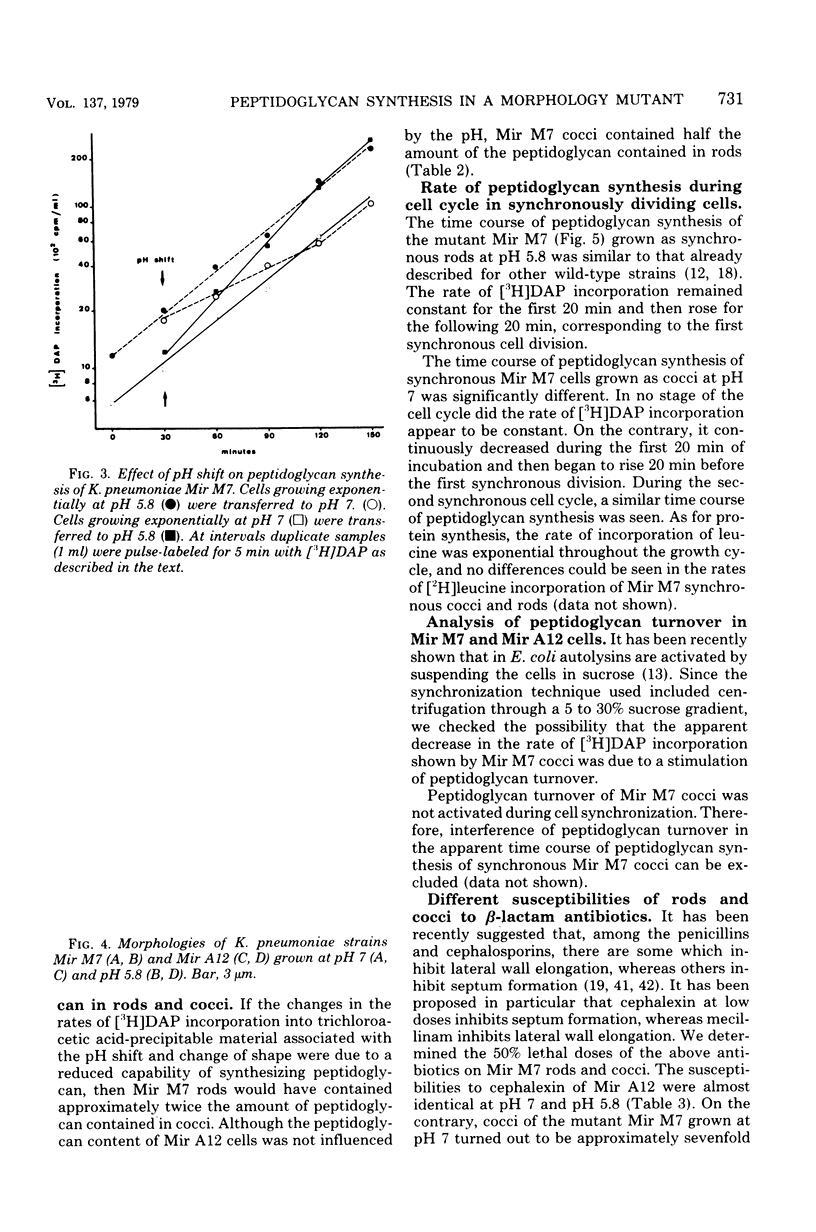
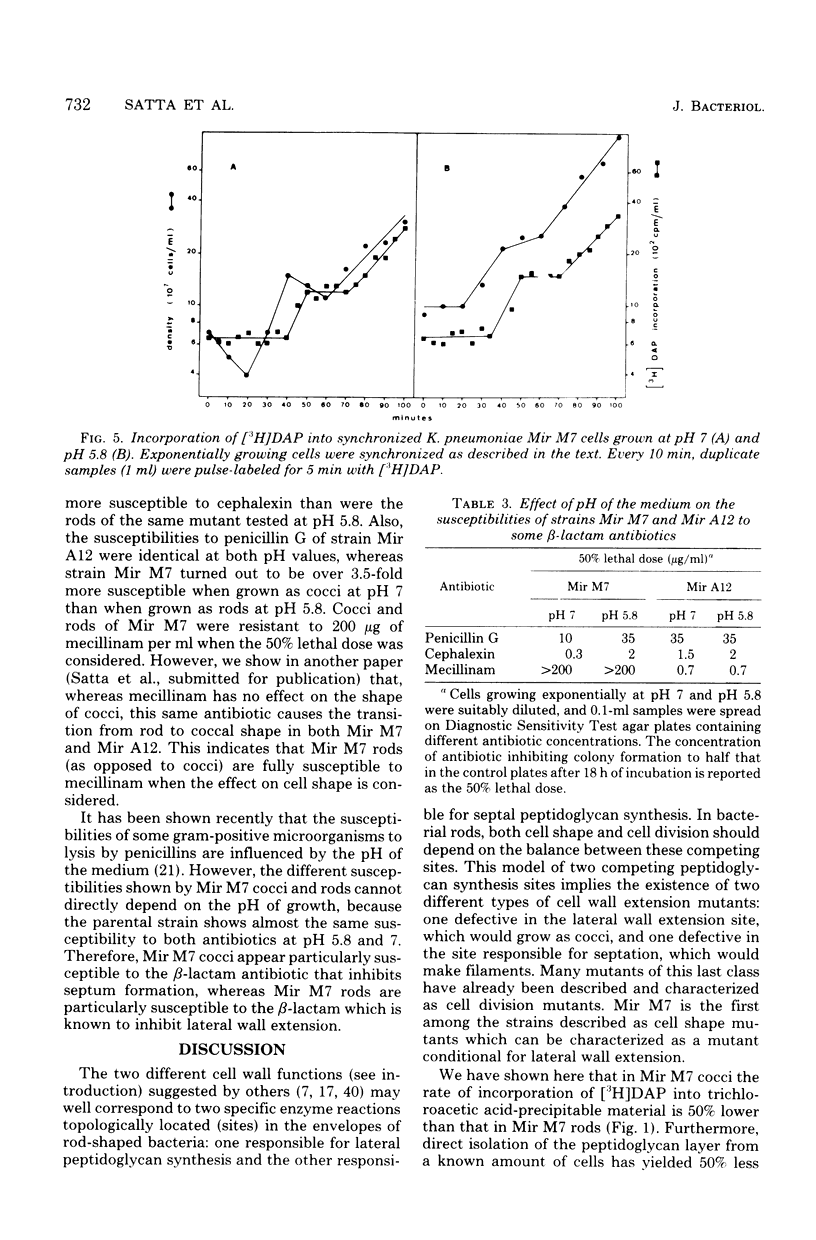
Mir M7 is a spontaneous morphologically conditional mutant of Klebsiella pneumoniae which grows as round cells (cocci) at pH 7 and as normal rods at pH 5.8. We studied the rates of peptidoglycan synthesis of cocci and rods growing at pH values of 7 and 5.8, respectively. It was found that exponentially growing cocci produced a reduced amount of peptidoglycan per cell, compared with rods. Moreover, a shift of cocci to the permissive pH (5.8) caused an increase in the rate of peptidoglycan synthesis, whereas the reverse shift of rods to pH 7 determined a twofold reduction in the rate of [3H]diaminopimelic acid incorporation. During synchronous growth at pH 7, the rate of peptidoglycan synthesis after cell division decreased with time and rose before and during the first division. The susceptibilities of rods and cocci to β-lactam antibiotics were also studied. It was found that cocci were more sensitive both to penicillin G and to cephalexin than were rods, but they showed a high level of resistance to mecillinam. The peculiar behavior of this mutant was interpreted as supporting the existence in bacterial rods of two different sites for peptidoglycan synthesis: one responsible for lateral wall elongation and one responsible for septum formation. In Mir M7, shape damage is described as dependent on the specific inhibition, at the nonpermissive pH, of the site for lateral wall extension.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boylan R. J., Mendelson N. H., Brooks D., Young F. E. Regulation of the bacterial cell wall: analysis of a mutant of Bacillus subtilis defective in biosynthesis of teichoic acid. J Bacteriol. 1972 Apr;110(1):281–290. doi: 10.1128/jb.110.1.281-290.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan R. J., Mendelson N. H. Initial characterization of a temperature-sensitive rod--mutant of Bacillus subtilis. J Bacteriol. 1969 Dec;100(3):1316–1321. doi: 10.1128/jb.100.3.1316-1321.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Wolff H. Attachment of lipoprotein to murein (peptidoglycan) of Escherichia coli in the presence and absence of penicillin FL 1060. J Bacteriol. 1975 Sep;123(3):888–897. doi: 10.1128/jb.123.3.888-897.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. N., Young F. E. Regulation of the bacterial cell wall: isolation and characterization of peptidoglycan mutants of Staphylococcus aureus. J Bacteriol. 1972 Jul;111(1):220–230. doi: 10.1128/jb.111.1.220-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana R., Satta G., Calegari L. The role of divalent cations in the rigid layer of morphological and conditional mutant of Klebsiella pneumoniae Mir M7. Ann Microbiol (Paris) 1974 Sep;125 B(2):275–292. [PubMed] [Google Scholar]
- Fujiwara T., Fukui S. Isolation of morphological mutants of Agrobacterium tumefaciens. J Bacteriol. 1972 May;110(2):743–746. doi: 10.1128/jb.110.2.743-746.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakenbeck R., Messer W. Oscillations in the synthesis of cell wall components in synchronized cultures of Escherichia coli. J Bacteriol. 1977 Mar;129(3):1234–1238. doi: 10.1128/jb.129.3.1234-1238.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann R., Bock-Hennig S. B., Schwarz U. Murein hydrolases in the envelope of Escherichia coli. Properties in situ and solubilization from the envelope. Eur J Biochem. 1974 Jan 3;41(1):203–208. doi: 10.1111/j.1432-1033.1974.tb03261.x. [DOI] [PubMed] [Google Scholar]
- Henning U. L. Determination of cell shape in bacteria. Annu Rev Microbiol. 1975;29:45–60. doi: 10.1146/annurev.mi.29.100175.000401. [DOI] [PubMed] [Google Scholar]
- Henning U., Rehn K., Braun V., Höhn B. Cell envelope and shape of Escherichia coli K12. Properties of a temperature-sensitive rod mutant. Eur J Biochem. 1972 Apr 24;26(4):570–586. doi: 10.1111/j.1432-1033.1972.tb01800.x. [DOI] [PubMed] [Google Scholar]
- Henning U., Rehn K., Hoehn B. Cell envelope and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2033–2036. doi: 10.1073/pnas.70.7.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Procaryotic cell division with respect to wall and membranes. CRC Crit Rev Microbiol. 1971 May;1(1):29–72. doi: 10.3109/10408417109104477. [DOI] [PubMed] [Google Scholar]
- Hoffmann B., Messer W., Schwarz U. Regulation of polar cap formation in the life cycle of Escherichia coli. J Supramol Struct. 1972;1(1):29–37. doi: 10.1002/jss.400010105. [DOI] [PubMed] [Google Scholar]
- James R., Haga J. Y., Pardee A. B. Inhibition of an early event in the cell division cycle of Escherichia coli by FL1060, an amidinopenicillanic acid. J Bacteriol. 1975 Jun;122(3):1283–1292. doi: 10.1128/jb.122.3.1283-1292.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazdunski C., Shaprio B. M. Relationship between permeability, cell division, and murein metabolism in a mutant of Escherichia coli. J Bacteriol. 1972 Aug;111(2):499–509. doi: 10.1128/jb.111.2.499-509.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez R., Ronda-Lain C., Tapia A., Waks S. B., Tomasz A. Suppression of the lytic and bactericidal effects of cell wallinhibitory antibiotics. Antimicrob Agents Chemother. 1976 Oct;10(4):697–706. doi: 10.1128/aac.10.4.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund F., Tybring L. 6 -amidinopenicillanic acids--a new group of antibiotics. Nat New Biol. 1972 Apr 5;236(66):135–137. doi: 10.1038/newbio236135a0. [DOI] [PubMed] [Google Scholar]
- Matsumoto H., Tazaki T. Genetic mapping of aro, pyr, and pur markers in Klebsiella pneumoniae. Jpn J Microbiol. 1971 Jan;15(1):11–20. [PubMed] [Google Scholar]
- Matsuzawa H., Hayakawa K., Sato T., Imahori K. Characterization and genetic analysis of a mutant of Escherichia coli K-12 with rounded morphology. J Bacteriol. 1973 Jul;115(1):436–442. doi: 10.1128/jb.115.1.436-442.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirelman D., Yashouv-Gan Y., Schwarz U. Peptidoglycan biosynthesis in a thermosensitive division mutant of Escherichia coli. Biochemistry. 1976 May 4;15(9):1781–1790. doi: 10.1021/bi00654a001. [DOI] [PubMed] [Google Scholar]
- Mirelman D., Yashouv-Gan Y., Schwarz U. Regulation of murein biosynthesis and septum formation in filamentous cells of Escherichia coli PAT 84. J Bacteriol. 1977 Mar;129(3):1593–1600. doi: 10.1128/jb.129.3.1593-1600.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normark S. Mutation in Escherichia coli K-12 mediating spherelike envelopes and changes tolerance to ultraviolet irradiation and some antibiotics. J Bacteriol. 1969 Jun;98(3):1274–1277. doi: 10.1128/jb.98.3.1274-1277.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olden K., Ito S., Wilson T. H. D-Alanine-requiring cell wall mutant of Escherichia coli. J Bacteriol. 1975 Jun;122(3):1310–1321. doi: 10.1128/jb.122.3.1310-1321.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. T., Burman L. FL-1060: a new penicillin with a unique mode of action. Biochem Biophys Res Commun. 1973 Apr 16;51(4):863–868. doi: 10.1016/0006-291x(73)90006-5. [DOI] [PubMed] [Google Scholar]
- Rodolakis A., Thomas P., Starka J. Morphological mutants of Escherichia coli. Isolation and ultrastructure of a chain-forming envC mutant. J Gen Microbiol. 1973 Apr;75(2):409–416. doi: 10.1099/00221287-75-2-409. [DOI] [PubMed] [Google Scholar]
- Rogers H. J., McConnell M., Burdett I. D. The isolation and characterization of mutants of Bacillus subtilis and Bacillus licheniformis with disturbed morphology and cell division. J Gen Microbiol. 1970 May;61(2):155–171. doi: 10.1099/00221287-61-2-155. [DOI] [PubMed] [Google Scholar]
- Rogers H. J., McConnell M., Hughes R. C. The chemistry of the cell walls of rod mutants of Bacillus subtilis. J Gen Microbiol. 1971 Jun;66(3):297–308. doi: 10.1099/00221287-66-3-297. [DOI] [PubMed] [Google Scholar]
- Rogers H. J., Thurman P. F., Taylor C., Reeve J. N. Mucopeptide synthesis by rod mutants of Bacillus subtilis. J Gen Microbiol. 1974 Dec;85(2):335–349. doi: 10.1099/00221287-85-2-335. [DOI] [PubMed] [Google Scholar]
- Satta G., Fontana R. Cell division, macromolecular synthesis and morphology dependent on the state of the envelope in a mutant of Klebsiella pneumoniae. J Gen Microbiol. 1974 Jan;80(1):65–75. doi: 10.1099/00221287-80-1-65. [DOI] [PubMed] [Google Scholar]
- Satta G., Fontana R. Characterization of a conditional mutant with altered envelope showing pH-dependent morphology and temperature-dependent division. J Gen Microbiol. 1974 Jan;80(1):51–63. doi: 10.1099/00221287-80-1-51. [DOI] [PubMed] [Google Scholar]
- Satta G., Pruzzo C., Debbia E., Fontana R. Close association between shape alteration and loss of immunity to superinfection in a wild-type Klebsiella pneumoniae stable lysogen which can be both immune and nonimmune to superinfection. J Virol. 1978 Dec;28(3):772–785. doi: 10.1128/jvi.28.3.772-785.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz U., Ryter A., Rambach A., Hellio R., Hirota Y. Process of cellular division in Escherichia coli: differention of growth zones in the Sacculus. J Mol Biol. 1975 Nov 15;98(4):749–759. doi: 10.1016/s0022-2836(75)80008-8. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Temperature-sensitive cell division mutants of Escherichia coli with thermolabile penicillin-binding proteins. J Bacteriol. 1977 Jul;131(1):293–305. doi: 10.1128/jb.131.1.293-305.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]




