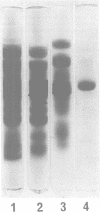
Abstract
Acetate kinase (EC 2.7.2.1) was purified from Acholeplasma laidlawii cytoplasm by a combination of ammonium sulfate fractionation, gel filtration, diethylaminoethyl-cellulose chromatography, and affinity chromatography on 8-(6-aminohexylamino)-adenosine 5′-triphosphate conjugated to Sepharose 4B. The enzyme was composed of polypeptide chains of about 50,000 molecular weight as estimated from sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Under nondenaturating conditions, apparent molecular weights between 64,000 and 130,000 were obtained, depending upon mainly the ionic strength of the test solution. The enzyme had a narrow specificity for phosphate acceptor acids, whereas both purine and pyrimidine nucleoside triphosphates were suitable phosphate donors. Na+ and K+ inhibited both acetyl phosphate and adenosine 5′-triphosphate synthesis, and the latter was also inhibited by high concentrations of adenosine 5′-diphosphate and acetyl phosphate. This substrate inhibition was partially abolished by 0.5 M NaCl. The enzyme catalyzed the independent adenosine 5′-diphosphate↔adenosine 5′-triphosphate and acetate↔acetyl phosphate exchanges. The rate of the latter was enhanced by the addition of cosubstrate Mg2+–adenosine 5′-triphosphate. The high affinity for substrates, except for acetate, indicated that under physiological conditions the direction of the enzymic reaction favors adenosine 5′-triphosphate synthesis. Thus, a mechanism for adenosine 5′-triphosphate generation in mycoplasmas is suggested.
Full text
PDF


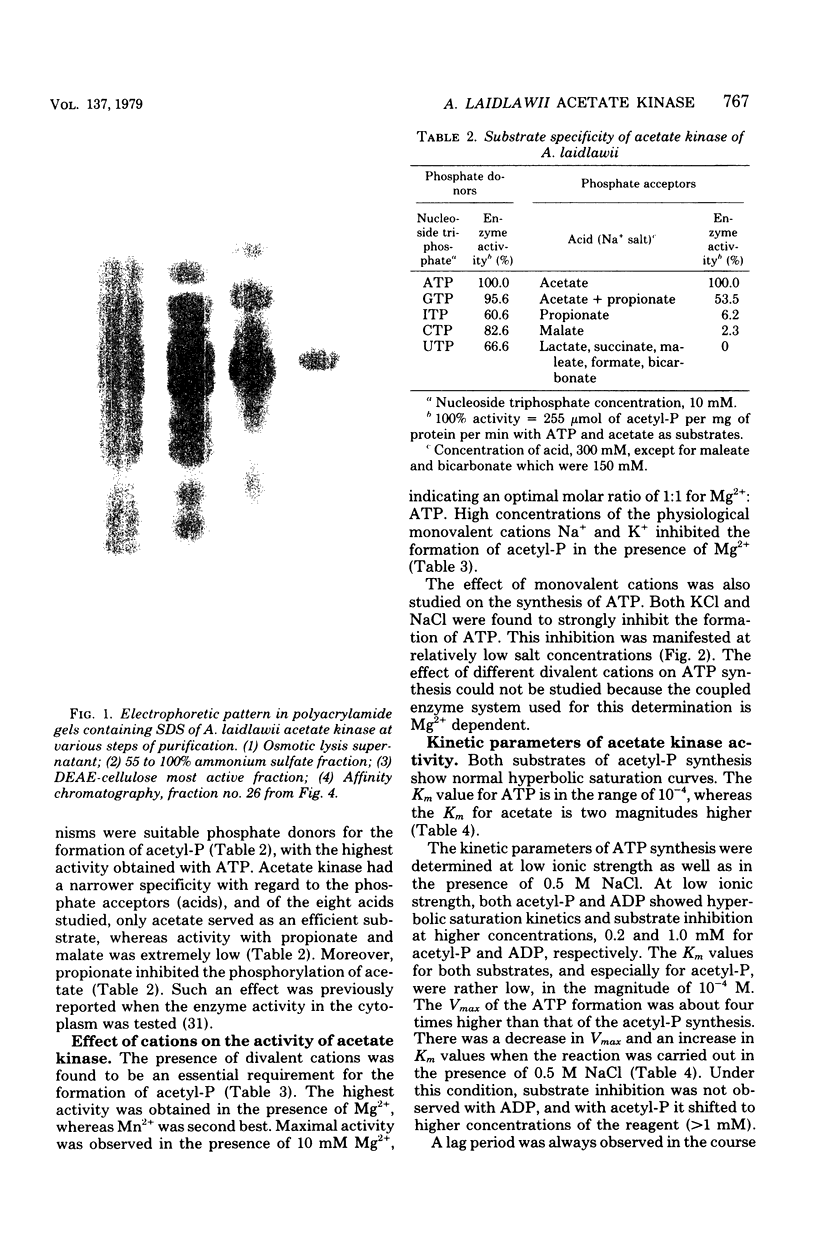
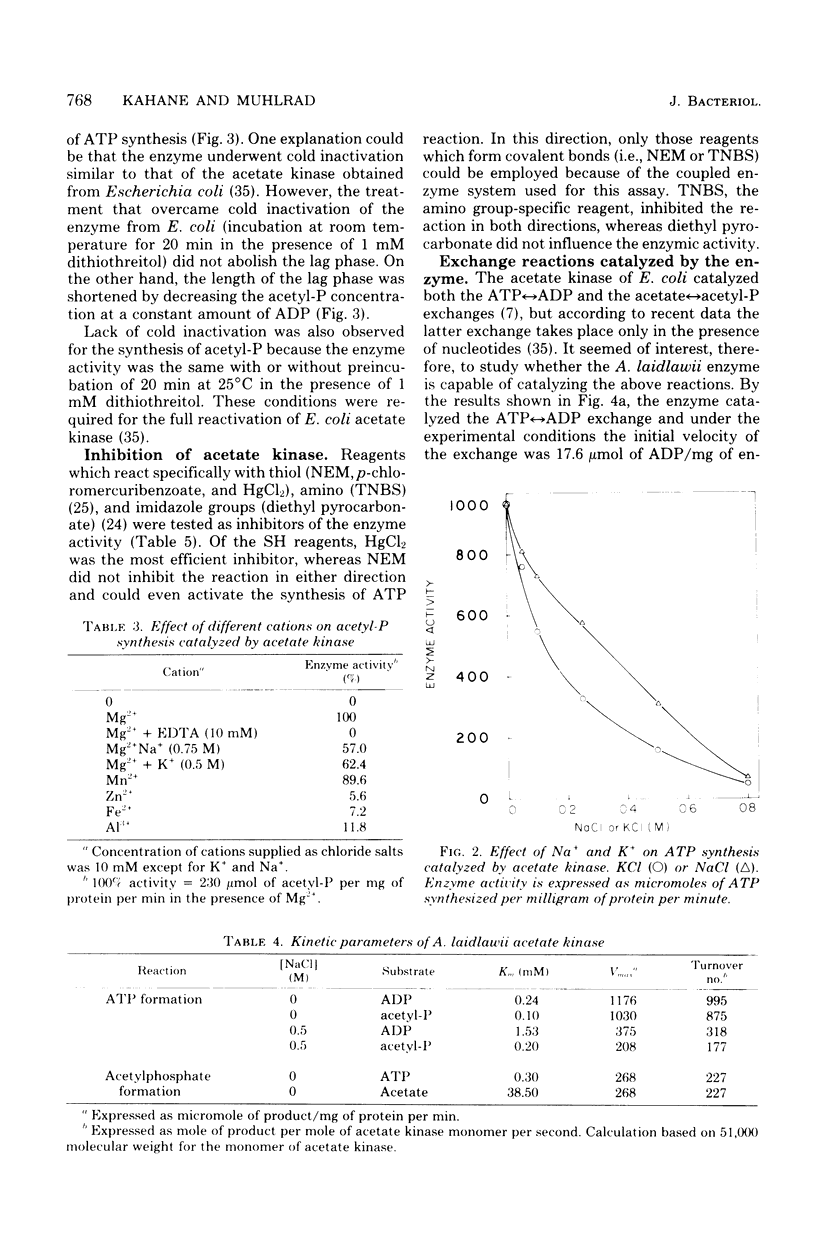

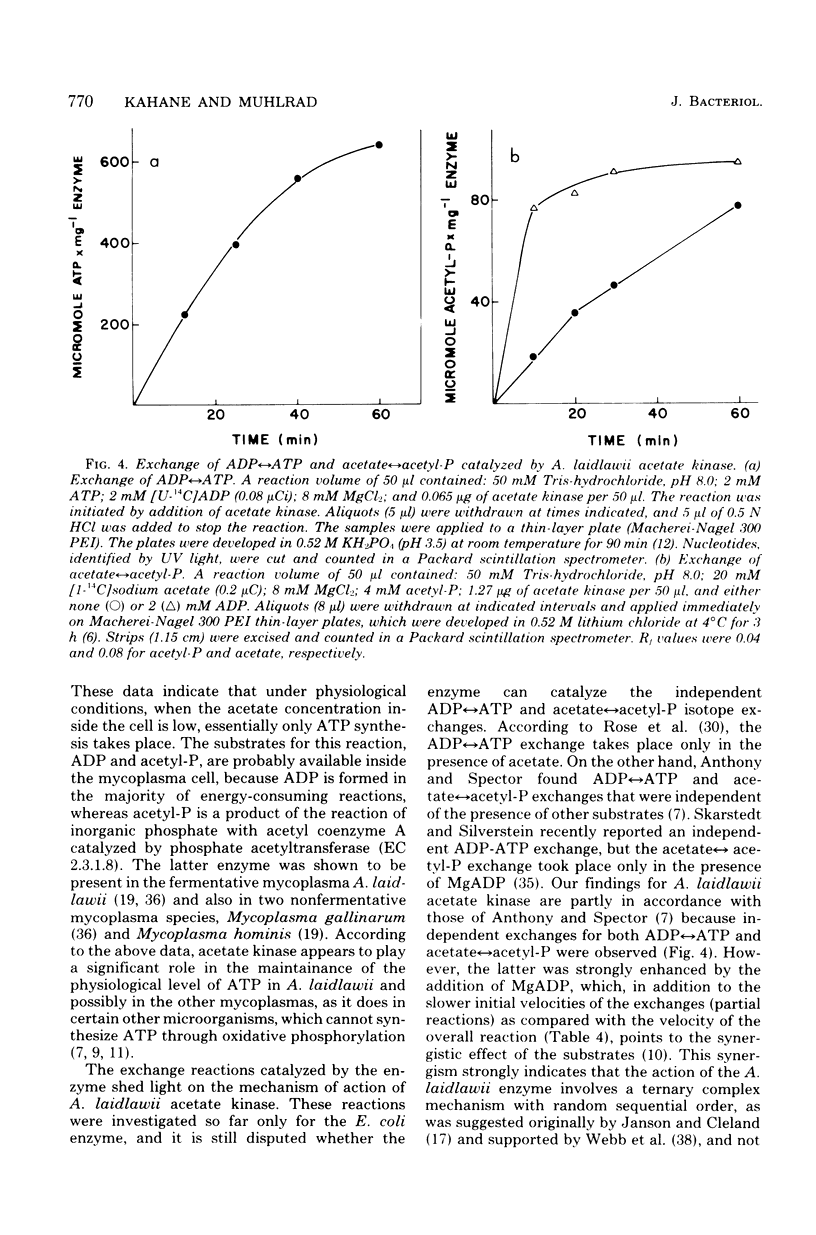


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN S. H., KELLERMEYER R. W., STJERNHOLM R. L., WOOD H. G. PURIFICATION AND PROPERTIES OF ENZYMES INVOLVED IN THE PROPIONIC ACID FERMENTATION. J Bacteriol. 1964 Jan;87:171–187. doi: 10.1128/jb.87.1.171-187.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrams A. The release of bound adenosine triphosphatase from isolated bacterial membranes and the properties of the solubilized enzyme. J Biol Chem. 1965 Sep;240(9):3675–3681. [PubMed] [Google Scholar]
- Amar A., Rottem S., Kahane I., Razin S. Characterization of the mycoplasma membrane proteins. VI. Composition and disposition of proteins in membranes from aging Mycoplasma hominis cultures. Biochim Biophys Acta. 1976 Mar 5;426(2):258–270. doi: 10.1016/0005-2736(76)90336-9. [DOI] [PubMed] [Google Scholar]
- Andreu J. M., Albendea J. A., Munõz E. Membrane adenosine triphosphatase of Micrococcus lysodeikticus. Molecular properties of the purified enzyme unstimulated by trypsin. Eur J Biochem. 1973 Sep 3;37(3):505–515. doi: 10.1111/j.1432-1033.1973.tb03012.x. [DOI] [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony R. S., Spector L. B. A phosphoenzyme intermediary in acetate kinase action. J Biol Chem. 1970 Dec 25;245(24):6739–6741. [PubMed] [Google Scholar]
- Anthony R. S., Spector L. B. Exchange reactions catalyzed by acetate kinase. J Biol Chem. 1971 Oct 25;246(20):6129–6135. [PubMed] [Google Scholar]
- Bowman C. M., Valdez R. O., Nishimura J. S. Acetate kinase from Veillonella alcalescens. Regulation of enzyme activity by succinate and substrates. J Biol Chem. 1976 May 25;251(10):3117–3121. [PubMed] [Google Scholar]
- Bridger W. A., Millen W. A., Boyer P. D. Substrate synergism and phosphoenzyme formation in catalysis by succinyl coenzyme A synthetase. Biochemistry. 1968 Oct;7(10):3608–3616. doi: 10.1021/bi00850a038. [DOI] [PubMed] [Google Scholar]
- Bárány M., Conover T. E., Schliselfeld L. H., Gaetjens E., Goffart M. Relation of properties of isolated myosin to those of intact muscles of the cat and sloth. Eur J Biochem. 1967 Sep;2(2):156–164. doi: 10.1111/j.1432-1033.1967.tb00120.x. [DOI] [PubMed] [Google Scholar]
- CASTREJON-DIEZ J., FISHER T. N., FISHER E., Jr Acetokinase reaction in several pleuropneumonia-like organisms. Biochem Biophys Res Commun. 1962 Nov 27;9:416–420. doi: 10.1016/0006-291x(62)90026-8. [DOI] [PubMed] [Google Scholar]
- Canale-Parola E. Physiology and evolution of spirochetes. Bacteriol Rev. 1977 Mar;41(1):181–204. doi: 10.1128/br.41.1.181-204.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashel M., Lazzarini R. A., Kalbacher B. An improved method for thin-layer chromatography of nucleotide mixtures containing 32P-labelled orthophosphate. J Chromatogr. 1969 Mar 11;40(1):103–109. doi: 10.1016/s0021-9673(01)96624-5. [DOI] [PubMed] [Google Scholar]
- Fenske J. D., Kenny G. E. Role of arginine deiminase in growth of Mycoplasma hominis. J Bacteriol. 1976 Apr;126(1):501–510. doi: 10.1128/jb.126.1.501-510.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holländer R., Wolf G., Mannheim W. Lipoquinones of some bacteria and mycoplasmas, with considerations on their functional significance. Antonie Van Leeuwenhoek. 1977;43(2):177–185. doi: 10.1007/BF00395672. [DOI] [PubMed] [Google Scholar]
- Janson C. A., Cleland W. W. The inhibition of acetate, pyruvate, and 3-phosphogylcerate kinases by chromium adenosine triphosphate. J Biol Chem. 1974 Apr 25;249(8):2567–2571. [PubMed] [Google Scholar]
- Kahane I., Greenstein S., Razin S. Carbohydrate content and enzymic activites in the membrane of Spiroplasma citri. J Gen Microbiol. 1977 Jul;101(1):173–176. doi: 10.1099/00221287-101-1-173. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamed R., Oplatka A. Applications of immobilized adenosine triphosphate in the study of myosin. Biochemistry. 1974 Jul 16;13(15):3137–3142. doi: 10.1021/bi00712a021. [DOI] [PubMed] [Google Scholar]
- Lee C. Y., Lazarus L. H., Kabakoff D. S., Russell P. J., Jr, Laver M., Kaplan N. O. Purification of kinases by general ligand affinity chromatography. Arch Biochem Biophys. 1977 Jan 15;178(1):8–18. doi: 10.1016/0003-9861(77)90165-5. [DOI] [PubMed] [Google Scholar]
- Masover G. K., Razin S., Hayflick L. Localization of enzymes in Ureaplasma urealyticum (T-strain mycoplasma). J Bacteriol. 1977 Apr;130(1):297–302. doi: 10.1128/jb.130.1.297-302.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelroy R. A., Whiteley H. R. Regulatory properties of acetokinase from Veillonella alcalescens. J Bacteriol. 1971 Jan;105(1):259–267. doi: 10.1128/jb.105.1.259-267.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAZIN S., MICHMANN J., SHIMSHONI Z. THE OCCURRENCE OF MYCOPLASMA (PLEUROPNEUMONIA-LIKE ORGANISMS, PPLO) IN THE ORAL CAVITY OF DENTULOUS AND EDENTULOUS SUBJECTS. J Dent Res. 1964 May-Jun;43:402–405. doi: 10.1177/00220345640430031101. [DOI] [PubMed] [Google Scholar]
- ROSE I. A., GRUNBERG-MANAGO M., KOREY S. R., OCHOA S. Enzymatic phosphorylation of acetate. J Biol Chem. 1954 Dec;211(2):737–756. [PubMed] [Google Scholar]
- Razin S. Physiology of mycoplasmas. Adv Microb Physiol. 1973;10:1–80. doi: 10.1016/s0065-2911(08)60086-7. [DOI] [PubMed] [Google Scholar]
- SCHIMKE R. T., BARILE M. F. ARGININE METABOLISM IN PLEUROPNEUMONIA-LIKE ORGANISMS ISOLATED FROM MAMMALIAN CELL CULTURE. J Bacteriol. 1963 Aug;86:195–206. doi: 10.1128/jb.86.2.195-206.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH P. F., HENRIKSON C. V. COMPARATIVE BIOSYNTHESIS OF MEVALONIC ACID BY MYCOPLASMA. J Bacteriol. 1965 Jan;89:146–153. doi: 10.1128/jb.89.1.146-153.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaupp A., Ljungdahl L. G. Purification and properties of acetate kinase from Clostridium thermoaceticum. Arch Microbiol. 1974;100(2):121–129. doi: 10.1007/BF00446312. [DOI] [PubMed] [Google Scholar]
- Schimke R. T., Berlin C. M., Sweeney E. W., Carroll W. R. The generation of energy by the arginine dihydrolase pathway in Mycoplasma hominis 07. J Biol Chem. 1966 May 25;241(10):2228–2236. [PubMed] [Google Scholar]
- Skarstedt M. T., Silverstein E. Escherichia coli acetate kinase mechanism studied by net initial rate, equilibrium, and independent isotopic exchange kinetics. J Biol Chem. 1976 Nov 10;251(21):6775–6783. [PubMed] [Google Scholar]
- THORNE K. J., JONES M. E. CARBAMYL AND ACETYL PHOSPHOKINASE ACTIVITIES OF STREPTOCOCCUS FAECALIS AND ESCHERICHIA COLI. J Biol Chem. 1963 Sep;238:2992–2998. [PubMed] [Google Scholar]
- Webb B. C., Todhunter J. A., Purich D. L. Studies on the kinetic mechanism and the phosphoryl-enzyme compound of the Escherichia coli acetate kinase reaction. Arch Biochem Biophys. 1976 Mar;173(1):282–292. doi: 10.1016/0003-9861(76)90261-7. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]