Abstract
Hereditary autosomal dominant myoclonus dystonia (MD) is a movement disorder characterized by involuntary lightning jerks and dystonic movements and postures alleviated by alcohol. Although various large families with MD have been described, no positive linkage has been found to a chromosomal location. We report a family with eight members with MD. Linkage analysis identified a 23-centimorgan region on chromosome 11q23 that cosegregates with the disease state (maximum multipoint logarithm of odds score = 2.96 at D11S897). This region contains an excellent candidate gene for involvement in the etiology of MD, the D2 dopamine receptor (DRD2) gene. Neurotransmission mediated by DRD2 is known to have a key role in the control of movement and also has been implicated in reward and reinforcement mechanisms and psychiatric disorders. Sequencing of the coding region of DRD2 indicated that all affected and obligate carriers were heterozygous for a Val154Ile change in exon 3 of the protein, which is highly conserved across species. This change was found neither in other unaffected members of the pedigree nor in 250 control chromosomes. Our finding provides evidence for the involvement of DRD2 in a disorder of the central nervous system and should lead to further insight into the function of the dopaminergic system in dystonia and other movement and mood disorders.
Myoclonus dystonia (MD), also known as hereditary essential myoclonus and myoclonic dystonia, is a rare movement disorder that can occur sporadically or in families with apparent autosomal dominant inheritance (1–3). It is characterized by onset of myoclonus in the first or second decade of life, frequently accompanied by dystonia involving the neck, arms, and face, a largely nonprogressive course with a normal life-span, an absence of seizures (4), and an alleviation of symptoms in response to alcohol but not to L-dopa (2). MD can be distinguished from early-onset primary torsion dystonia, which also can cause myoclonic jerks (3, 5, 6). A 3-bp deletion in the DYT1 gene causes the majority of cases of early-onset primary torsion dystonia (7), and linkage to the DYT1 locus has been excluded in several families with MD (8, 9), including the one under study (data not shown). Despite the description of various large families with MD in the literature (3, 8–13), as yet, no positive linkage has been reported to a chromosomal location.
Taking a candidate-gene approach, we determined that the D2 dopamine receptor (DRD2) gene seemed to be a particularly attractive candidate to be involved in the etiology of MD. DRD2 is highly expressed in the basal ganglia (14), which is a control center for movement, and drugs that block dopamine receptors can lead to dystonic symptoms (15). Patients with juvenile parkinsonism frequently present with dystonia (16); also, some forms of early-onset dystonia are believed to involve dopaminergic transmission, e.g., dopa-responsive dystonia with mutations in genes encoding enzymes in dopamine synthesis (17, 18) and early-onset primary torsion dystonia, in which the transcript for the responsible gene, DYT1, is expressed at high levels in dopaminergic neurons of the substantia nigra (19). In addition, the DRD2 receptor is believed to have a role in affect, emotion, and reward mechanisms (14) and has been implicated in the etiology of alcoholism (20, 21) and schizophrenia (22). Interestingly, many patients with MD consume large quantities of alcohol (8), frequently exceeding the amount necessary to relieve the myoclonus, or even to be addicted to alcohol or drugs (11). Some patients, including several members of the family under study, also display features of psychiatric disorders/affective traits such as anxiety, neurosis, phobias (11), depression, and panic attacks (M.F.B. and S.B., unpublished observations).
MATERIALS AND METHODS
Subjects.
After giving informed consent, all participants underwent a standardized neurological examination. The diagnosis of MD was established according to published criteria (2–4), including videotaped examinations reviewed by neurologists trained in movement disorders who were blind to patient status or relationship.
Haplotype Analysis.
Genotyping was carried out with the simple sequence repeat DNA markers D11S2002–8.9–D11S1367–13.7–D11S1302–0.0–D11S2000–1.7–D11S4961–3.4–DRD2–0.5–D11S897–0.0–D11S1986–3.4–D11S1992–5.4–D11S1998 [methods specified by Research Genetics, Huntsville, AL (www.resgen.com); marker distances according to Marshfield Medical Research, Marshfield, WI (www.marshmed.org/genetics)]. PCR products were analyzed on a Li-Cor (Lincoln, NE) automated sequencer or detected by autoradiography.
Linkage Analysis.
We conducted pairwise and multipoint linkage analyses by using the vitesse program (23). Disease allele frequency was estimated to be 0.01%. We specified an age correction, based on the age-of-onset distribution within this family, in which all affected individuals had onset before the age of 16 years. Maximum penetrance was estimated at a conservative level of 50%. Marker allele frequencies were assumed to be equal.
DNA Sequence Analysis.
Dideoxy cycle sequencing of PCR products amplified from genomic DNA was performed with the AmpliSequence sequencing kit (Perkin–Elmer) after enzymatic clean up with exonuclease I and shrimp alkaline phosphatase (United States Biochemical). The following primers were used for PCR amplification and sequencing of exon 3 of DRD2: DRD23F (5′-GGTGTGTGCATATTGTGGAG-3′) and DRD23R (5′-TGCCAGGACTCTGTTCCCTC-3′). PCR was carried out at 94°C for 1 min, 48°C for 1 min, and 72°C for 1 min (40 cycles). DRD2 primer sequences for exons 1, 2, and 4–7 as well as PCR conditions are available on request.
RESULTS AND DISCUSSION
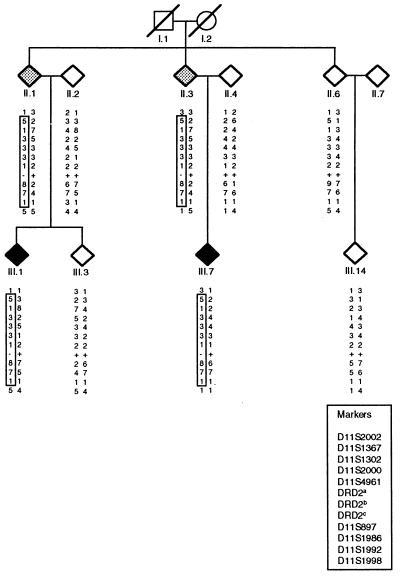
We studied a 30-member family of Welsh–Scottish–German origin with 8 individuals showing alcohol-responsive MD (5 men; 3 women; mean age of onset = 8.1 ± 4.7 years; range: 2–16 years) and 2 unaffected obligate carriers. Clinical features included predominant myoclonus in three, dystonia in one, and features of both in four individuals. Of the affected individuals, three reported less severe myoclonic symptoms after ingestion of alcohol; there was no history of alcohol abuse. Affective traits, including depression, manic-depression, anxiety, panic attacks, and obsessive-compulsive disorder were reported by history in five of the affected individuals and one obligate carrier. The mode of transmission suggested autosomal dominant inheritance with reduced penetrance. Strong evidence for linkage was found for markers on the long arm of chromosome 11, with a maximum two-point logarithm of odds (lod) score at D11S897 (z = 2.96; Table 1). Multipoint analysis with a subset of these markers confirmed that the likelihood of linkage is greatest at D11S897 (maximum multipoint lod score was 2.96). The lod score of 2.96 closely approximates the maximum potential lod score for this particular family size and structure. Critical recombination events between markers D11S2002 and D11S1367 and between D11S1992 and D11S1998 occurred in individuals II.1 or II.3 (see Fig. 1), which would place the MD locus within a region of about 23 centimorgans. This chromosomal region, 11q23, contains a number of genes that are known to play a role in functions and/or diseases of the central nervous system, including the neural cell-adhesion molecule gene (24), the ataxia telangiectasia gene (25), and the DRD2 gene (26, 27).
Table 1.
Results of two-point linkage analysis with chromosome 11 markers in the family with myoclonus dystonia
| Marker | lod score, θ
|
|||||
|---|---|---|---|---|---|---|
| 0.00 | 0.01 | 0.05 | 0.10 | 0.20 | 0.30 | |
| D11S2002 | −3.32 | −0.84 | −0.21 | −0.01 | 0.10 | 0.08 |
| D11S1367 | 2.66 | 2.61 | 2.42 | 2.16 | 1.63 | 1.06 |
| D11S1302 | 2.66 | 2.61 | 2.41 | 2.16 | 1.63 | 1.06 |
| D11S2000 | 2.36 | 2.31 | 2.14 | 1.91 | 1.43 | 0.92 |
| D11S4961 | 0.27 | 0.27 | 0.25 | 0.24 | 0.19 | 0.14 |
| DRD2 | 0.91 | 0.90 | 0.82 | 0.73 | 0.55 | 0.36 |
| D11S897 | 2.96 | 2.91 | 2.69 | 2.41 | 1.81 | 1.15 |
| D11S1986 | 2.58 | 2.54 | 2.35 | 2.11 | 1.60 | 1.05 |
| D11S1992 | 0.28 | 0.27 | 0.26 | 0.24 | 0.20 | 0.14 |
| D11S1998 | −1.95 | 0.25 | 0.81 | 0.93 | 0.84 | 0.59 |
Figure 1.
Partial pedigree of the family with MD. Affected individuals are shaded in black, and obligate carriers are in gray. Haplotypes are given at tested markers in the 11q22–11q23 region. Boxed haplotypes cosegregate with the disease/carrier status. DRD2a denotes the polymorphic repeat marker located within intron 2 of the DRD2 gene (39); DRD2b indicates the known, silent polymorphism in exon 3 (1, polymorphism present; 2, polymorphism not present) of the DRD2 gene (28) that cosegregates with the G → A sequence change in exon 3 in affected individuals (denoted DRD2c; +, the normal sequence of G; −, altered sequence A).
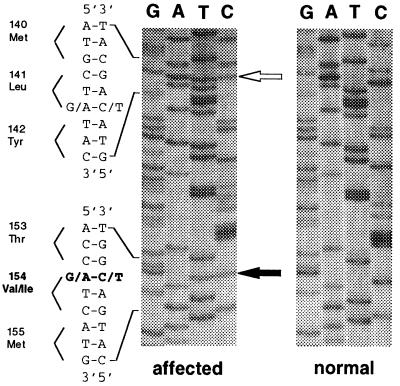
DNA sequence analysis of the coding region of DRD2 showed all affected family members and obligate carriers to be heterozygous for a G → A transition in the first base of codon 154, causing a Val154Ile change in a highly conserved region of exon 3 (Fig. 2). This sequence change was in complete linkage with a silent polymorphism (Leu141Leu; Fig. 2) known to be present in about 4% of the white population (28). The Val154Ile change was not found in any of 250 control chromosomes of the Centre d’Étude du Polymorphisme Humain (Paris), whereas seven control individuals (5.6%) were heterozygous for the Leu141Leu polymorphism.
Figure 2.
Sequence of exon 3 of the D2 dopamine receptor. All affected individuals and obligate carriers show a heterozygous Val154Ile change caused by a G → A transition in the first base of codon 154 (indicated by the black arrow). This mutation cosegregates with a G → A transition in the third base of codon 141 resulting in a silent Leu141Leu change (indicated by the open arrow). (Right) For comparison, the normal sequence is shown. (The reverse sequence is shown.)
Our data show that a sequence change in DRD2 is associated with MD in all affected family members and obligate gene carriers. Individual II.6 seems to share at least part of the disease-associated haplotype with II.1 and II.3. Individual II.6 has a 9 instead of the 8 allele at marker D11S897, which could be explained by “slippage” or a recombination event at some point above or below the DRD2 locus. Supporting a recombination event above the DRD2 locus, II.6 does not carry the mutation or the polymorphism in exon 3 of the DRD2 gene and, therefore, is assumed to have inherited this part of chromosome 11 from the normal chromosome in the carrier.
The issue of possible anticipation is raised in our family, because individuals II.1 and II.3 have never shown any signs of MD and thus are nonmanifesting carriers of the mutation. Mean age of onset of the six affected family members in generation III was 9.8 ± 4.0 years, and mean age of onset of MD in the two affected individuals in generation IV was 3 and 4 years. However, this issue is difficult to address in a single family. Also, reduced penetrance (3) but not anticipation has been reported in other families with MD (3, 8, 10). Age of onset in subsequent generations is likely to be biased by increased awareness of the symptoms with earlier diagnosis of the disease. Therefore, unaffected status of individuals II.1 and II.3 could be explained by incomplete penetrance alone.
This same variation in DRD2 was not detected in other individuals with MD, including five sporadic patients and patients from two families with multiple affected members (not suitable for linkage), nor could linkage to the 11q23 region be found in three other MD families, suggesting that mutations in other gene(s) or other mutations in DRD2 may underlie the MD phenotype. Similar clinical phenotypes caused by different genes have been described in many other neurological disorders, including the hereditary dystonias, for which an increasing number of genes have been mapped and identified (for review see ref. 29). As shown by the example of Parkinson’s disease, mutations in the α-synuclein gene (30) are an extremely rare cause of familial Parkinson’s disease and were not found in hundreds of sporadic or familial patients tested (31). Likewise, the Ile93Met change in the ubiquitin carboxyl-terminal hydrolase L1 gene was present only in 1 of 72 families with Parkinson’s disease studied (32). Among further possible MD candidate proteins are the other dopamine receptors and proteins involved in second messenger pathways, such as G protein subunits that interact with dopamine receptors.
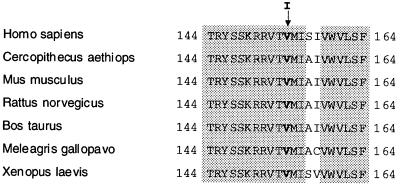
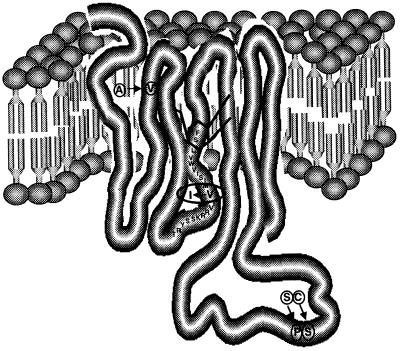
Although, at first sight, the V → I change in DRD2 in our family seems to be minor, a number of facts point toward its significance. (i) The mutation occurs in a region that is highly conserved in DRD2 across species including Cercopithecus aethiops, Mus musculus, Rattus norvegicus, Bos taurus, Meleagris gallopavo, and Xenopus laevis (Fig. 3). (ii) This change has never been reported to occur as a normal polymorphism, although extensive screening for mutations of DRD2 has been performed in controls and patients with many diseases (e.g., schizophrenia and alcoholism; refs. 28 and 33), and was not found in this study in any of the 250 control chromosomes tested. (iii) The change in amino acid is located in a transmembrane domain, and, in general, transmembrane domains are of particular importance for agonist binding (34). The location of the mutated valine close to the inner surface of the cell membrane and the third cytoplasmic loop (Fig. 4) predicts possible interference with receptor/G protein interaction. This prediction is supported by mutagenesis studies of DRD2 suggesting that certain hydrophobic residues in this region are important for both ligand binding and receptor activation (35). (iv) Single-base-pair mutations causing amino acid substitutions in other G protein-coupled receptors have been shown to cause disease. For example, the A → G (Gly578Asp) change in the sixth transmembrane domain of the luteinizing hormone receptor results in male precocious puberty (36). (v) Other changes in similarly uncharged amino acids can have major effects. For example, the L → V mutation in the AREA DNA binding domain of Aspergillus nidulans leads to dramatic changes in the expression profile of genes controlled by this transcription factor (37), and an A → V substitution in exon 7 of the follicle-stimulating hormone receptor gene results in suppression of spermatogenesis and of fertility in men homozygous for this change (38).
Figure 3.
Comparison of predicted amino acid sequences of DRD2 in different species. Shaded residues are identical in all species. The mutated valine residue at position 154 is in bold.
Figure 4.
Stuctural model of DRD2. The ring structure represents a dopamine molecule located in its binding site. Known polymorphisms are circled (40). The V → I change at the beginning of the fourth transmembrane domain, facing the inner surface of the cell membrane, is indicated by the oval.
Dopamine is the predominant catecholamine neurotransmitter in the central nervous system (14). Disturbances of the dopaminergic system are relevant in the etiology of various movement disorders, such as Parkinson’s disease, the dopa-responsive dystonias, and early-onset torsion dystonia. Among the five dopamine receptors (D1–D5) identified so far, DRD2 is a particularly attractive candidate to cause MD, as it is known to control various functions of the central nervous system, including movement, positive reinforcement/addiction, and possibly emotion (14), all of which are affected to a certain extent in at least some patients with MD. Studies of the function of the mutated DRD2 in comparison to the normal should help to elucidate the role of DRD2 and dopaminergic neurotransmission in MD, as well as in other movement and mood disorders.
Acknowledgments
We are grateful to the patients and family members who participated in this study and would like to thank Drs. S. Fink, J. Friedman, R. Kurlan, A. Lang, and P. Vieregge for providing blood samples of individuals with MD. This work was supported by a grant from the Dystonia Medical Research Foundation (to M.F.B., P.L.K., D.d.L., S.F., X.O.B., and L.J.O.), by National Institute of Neurological Disorders and Stroke Grant NS28384 (to X.O.B.), by a grant from the Bachmann–Strauss Foundation (to M.F.B.), and by a grant from the Deutsche Forschungsgemeinschaft (to C.K.).
ABBREVIATIONS
- MD
myoclonus dystonia
- lod
logarithm of odds
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Gasser T. Adv Neurol. 1998;78:325–334. [PubMed] [Google Scholar]
- 2.Quinn N P. Movement Disorders. 1996;11:119–124. doi: 10.1002/mds.870110202. [DOI] [PubMed] [Google Scholar]
- 3.Quinn N P, Rothwell J C, Thompson P D, Marsden C D. Adv Neurol. 1988;50:391–401. [PubMed] [Google Scholar]
- 4.Mahloudji M, Pikielny R T. Brain. 1967;90:669–674. doi: 10.1093/brain/90.3.669. [DOI] [PubMed] [Google Scholar]
- 5.Bressman S B, de Leon D, Kramer P L, Ozelius L J, Brin M F, Greene P E, Fahn S, Breakefield X O, Risch N J. Ann Neurol. 1994;36:771–777. doi: 10.1002/ana.410360514. [DOI] [PubMed] [Google Scholar]
- 6.Obeso J A, Rothwell J C, Lang A E, Marsden C D. Neurology. 1983;33:825–830. doi: 10.1212/wnl.33.7.825. [DOI] [PubMed] [Google Scholar]
- 7.Ozelius L J, Hewett J W, Page C E, Bressman S B, Kramer P L, Shalish C, de Leon D, Brin M F, Raymond D, Corey D P, et al. Nat Genet. 1997;17:40–48. doi: 10.1038/ng0997-40. [DOI] [PubMed] [Google Scholar]
- 8.Gasser T, Bereznai B, Müller B, Pruszak-Seel R, Damrich R, Deuschl G, Oertel W H. Movement Disorders. 1996;11:363–370. doi: 10.1002/mds.870110403. [DOI] [PubMed] [Google Scholar]
- 9.Wahlström J, Ozelius L, Kramer P, Kyllerman M, Schuback D, Forsgren L, Holmgren G, Drugge U, Sanner G, Fahn S, et al. Clin Genet. 1994;45:88–92. doi: 10.1111/j.1399-0004.1994.tb04000.x. [DOI] [PubMed] [Google Scholar]
- 10.Kurlan R, Behr J, Miller C, Shoulson I. Ann Neurol. 1985;18:162–163. [Google Scholar]
- 11.Kyllerman M, Forsgren L, Sanner G, Holmgren G, Wahlström J, Drugge U. Movement Disorders. 1990;5:270–279. doi: 10.1002/mds.870050403. [DOI] [PubMed] [Google Scholar]
- 12.Przuntek H, Muhr H. J Neurol. 1983;230:153–162. doi: 10.1007/BF00313626. [DOI] [PubMed] [Google Scholar]
- 13.Fahn S, Sjaastad O. Movement Disorders. 1991;6:237–247. doi: 10.1002/mds.870060308. [DOI] [PubMed] [Google Scholar]
- 14.Missale C, Nash S R, Robinson S W, Jaber M, Caron M G. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 15.LeWitt P A. In: Handbook of Dystonia. King J, Tsui C, Calne D B, editors. New York: Dekker; 1995. pp. 227–240. [Google Scholar]
- 16.Ishikawa A, Tsuji S. Neurology. 1996;47:160–166. doi: 10.1212/wnl.47.1.160. [DOI] [PubMed] [Google Scholar]
- 17.Ichinose H, Ohye T, Takahashi E, Seki N, Hori T, Segawa M, Nomura Y, Endo K, Tanaka H, Tsuji S, et al. Nat Genet. 1994;8:236–242. doi: 10.1038/ng1194-236. [DOI] [PubMed] [Google Scholar]
- 18.Knappskog P M, Flatmark T, Mallet J, Lüdecke B, Bartholome K. Hum Mol Genet. 1995;4:1209–1212. doi: 10.1093/hmg/4.7.1209. [DOI] [PubMed] [Google Scholar]
- 19.Augood S J, Penney J B, Jr, Friberg I K, Breakefield X O, Young A B, Ozelius L J, Standaert D G. Ann Neurol. 1998;43:669–673. doi: 10.1002/ana.410430518. [DOI] [PubMed] [Google Scholar]
- 20.Noble E P, Zhang X, Ritchie T, Lawford B R, Grosser S C, Young R M, Sparkes R S. Psychiatry Res. 1998;81:133–147. doi: 10.1016/s0165-1781(98)00084-5. [DOI] [PubMed] [Google Scholar]
- 21.Blum K, Noble E P, Sheridan P J, Montgomery A, Ritchie T, Ozkaragoz T, Fitch R J, Wood R, Finley O, Sadlack F. Alcohol. 1993;10:59–67. doi: 10.1016/0741-8329(93)90054-r. [DOI] [PubMed] [Google Scholar]
- 22.Arinami T, Gao M, Hamaguchi H, Toru M. Hum Mol Genet. 1997;6:577–582. doi: 10.1093/hmg/6.4.577. [DOI] [PubMed] [Google Scholar]
- 23.O’Connell J R, Weeks D E. Nat Genet. 1995;11:402–408. doi: 10.1038/ng1295-402. [DOI] [PubMed] [Google Scholar]
- 24.Eubanks J H, Djabali M, Selleri L, Grandy D K, Civelli O, McElligott D L, Evans G A. Genomics. 1992;14:1010–1018. doi: 10.1016/s0888-7543(05)80124-7. [DOI] [PubMed] [Google Scholar]
- 25.Gatti R A, Berkel I, Boder E, Braedt G, Charmley P, Concannon P, Ersoy F, Foroud T, Jaspers N G, Lange K, et al. Nature (London) 1988;336:577–580. doi: 10.1038/336577a0. [DOI] [PubMed] [Google Scholar]
- 26.Grandy D K, Litt M, Allen L, Bunzow J R, Marchionni M, Makam H, Reed L, Magenis R E, Civelli O. Am J Hum Genet. 1989;45:778–785. [PMC free article] [PubMed] [Google Scholar]
- 27.Grandy D K, Marchionni M A, Makam H, Stofko R E, Alfano M, Frothingham L, Fischer J B, Burke-Howie K J, Bunzow J R, Server A C, et al. Proc Natl Acad Sci USA. 1989;86:9762–9766. doi: 10.1073/pnas.86.24.9762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarkar G, Kapelner S, Grandy D K, Marchionni M, Civelli O, Sobell J, Heston L, Sommer S S. Genomics. 1991;11:8–14. doi: 10.1016/0888-7543(91)90096-w. [DOI] [PubMed] [Google Scholar]
- 29.Müller U, Steinberger D, Nemeth A H. Neurogenetics. 1998;1:165–177. doi: 10.1007/s100480050025. [DOI] [PubMed] [Google Scholar]
- 30.Polymeropoulos M H, Lavedan C, Leroy E, Ide S E, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, et al. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 31.Golbe L I. Movement Disorders. 1999;14:6–9. doi: 10.1002/1531-8257(199901)14:1<6::aid-mds1004>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 32.Leroy E, Boyer R, Auburger G, Leube B, Ulm G, Mezey E, Harta G, Brownstein M, Jonnalagada S, Chernova T, et al. Nature (London) 1998;395:451–452. doi: 10.1038/26652. [DOI] [PubMed] [Google Scholar]
- 33.Gejman P V, Ram A, Gelernter J, Friedman E, Cao Q, Pickar D, Blum K, Noble E P, Kranzler H R, O’Malley S, et al. J Am Med Assoc. 1994;271:204–208. [PubMed] [Google Scholar]
- 34.Javitch J A, Ballesteros J A, Weinstein H, Chen J. Biochemistry. 1998;37:998–1006. doi: 10.1021/bi972241y. [DOI] [PubMed] [Google Scholar]
- 35.Cho W, Taylor L P, Mansour A, Akil H. J Neurochem. 1995;65:2105–2115. doi: 10.1046/j.1471-4159.1995.65052105.x. [DOI] [PubMed] [Google Scholar]
- 36.Shenker A, Laue L, Kosugi S, Merendino J J, Minegishi T, Cutler G B. Nature (London) 1993;365:652–654. doi: 10.1038/365652a0. [DOI] [PubMed] [Google Scholar]
- 37.Starich M R, Wikström M, Schumacher S, Arst H N, Jr, Gronenborn A M, Clore G M. J Mol Biol. 1998;277:621–634. doi: 10.1006/jmbi.1997.1626. [DOI] [PubMed] [Google Scholar]
- 38.Tapanainen J S, Aittomaki K, Min J, Vaskivuo T, Huhtaniemi I T. Nat Genet. 1997;15:205–206. doi: 10.1038/ng0297-205. [DOI] [PubMed] [Google Scholar]
- 39.Hauge X Y, Grandy D K, Eubanks J H, Evans G A, Civelli O, Litt M. Genomics. 1991;10:527–530. doi: 10.1016/0888-7543(91)90431-d. [DOI] [PubMed] [Google Scholar]
- 40.Seeman P. Sci Am. 1995;2(5):28–37. [Google Scholar]