Abstract
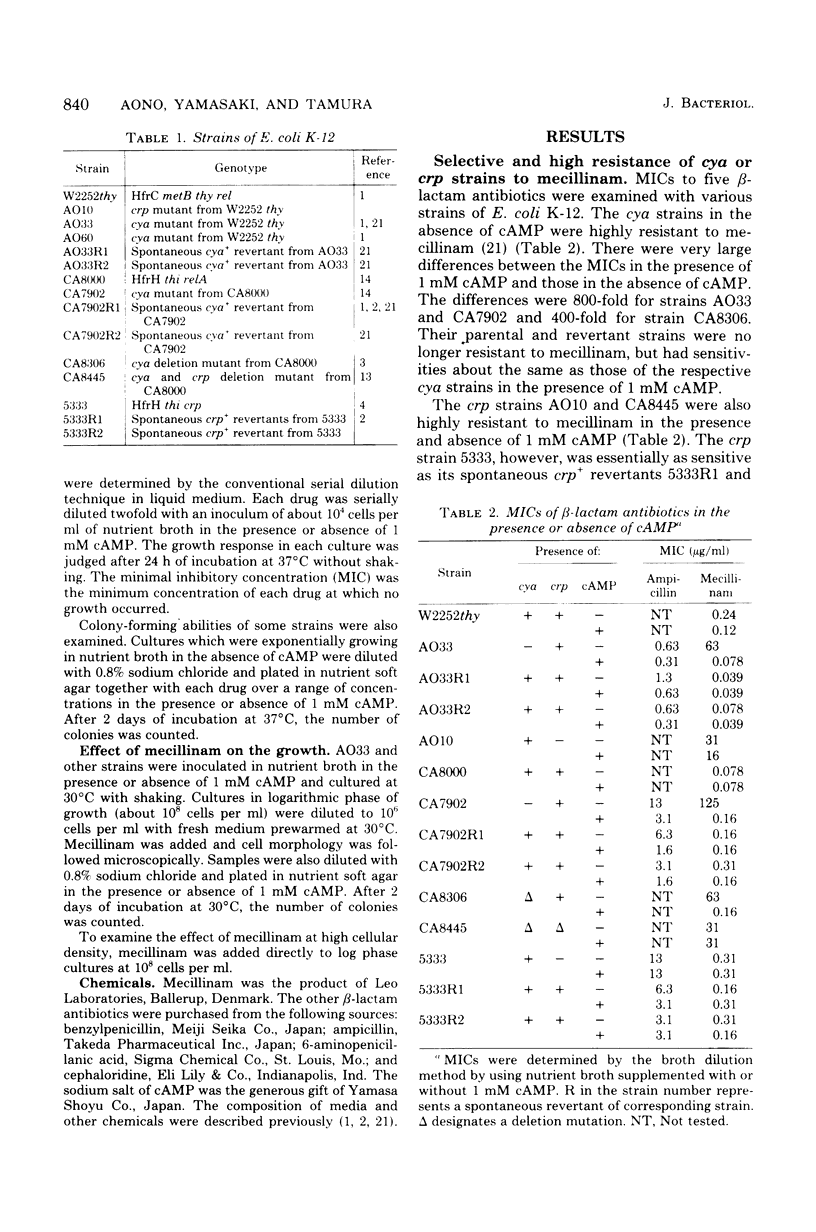
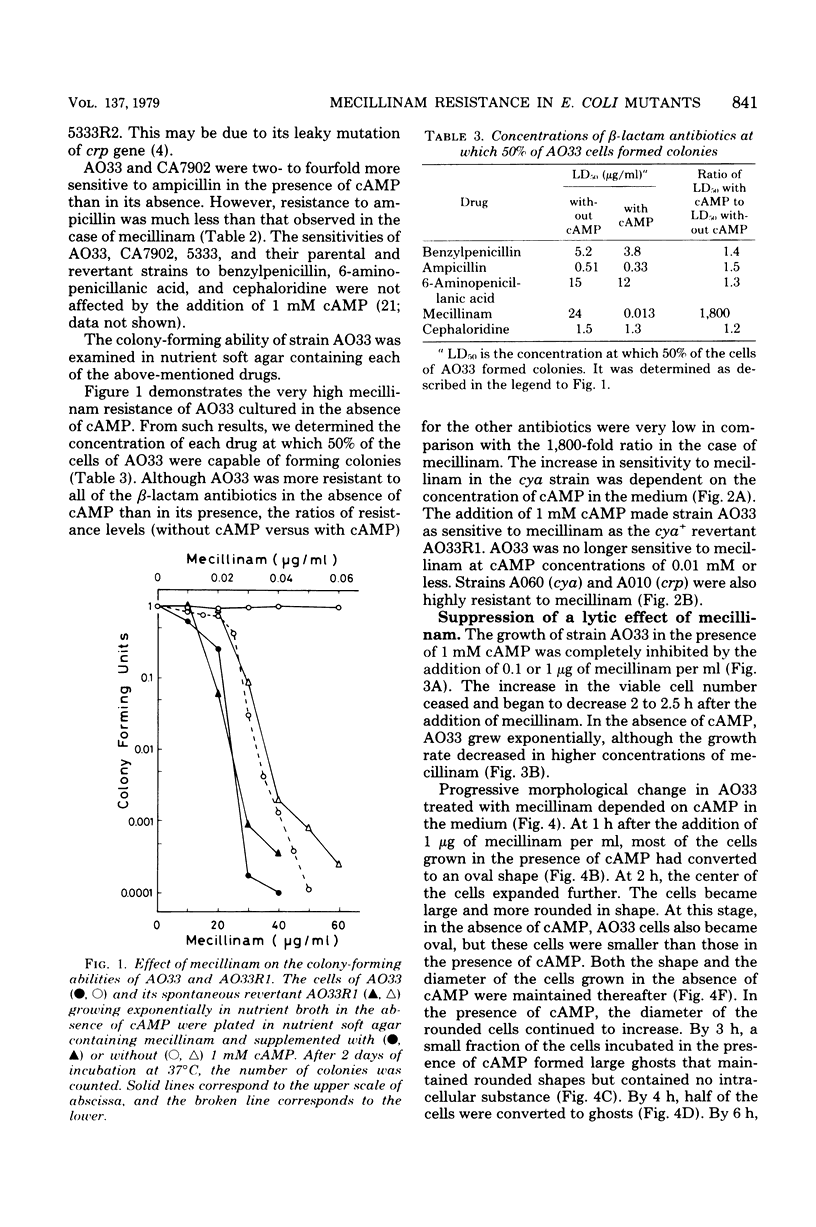
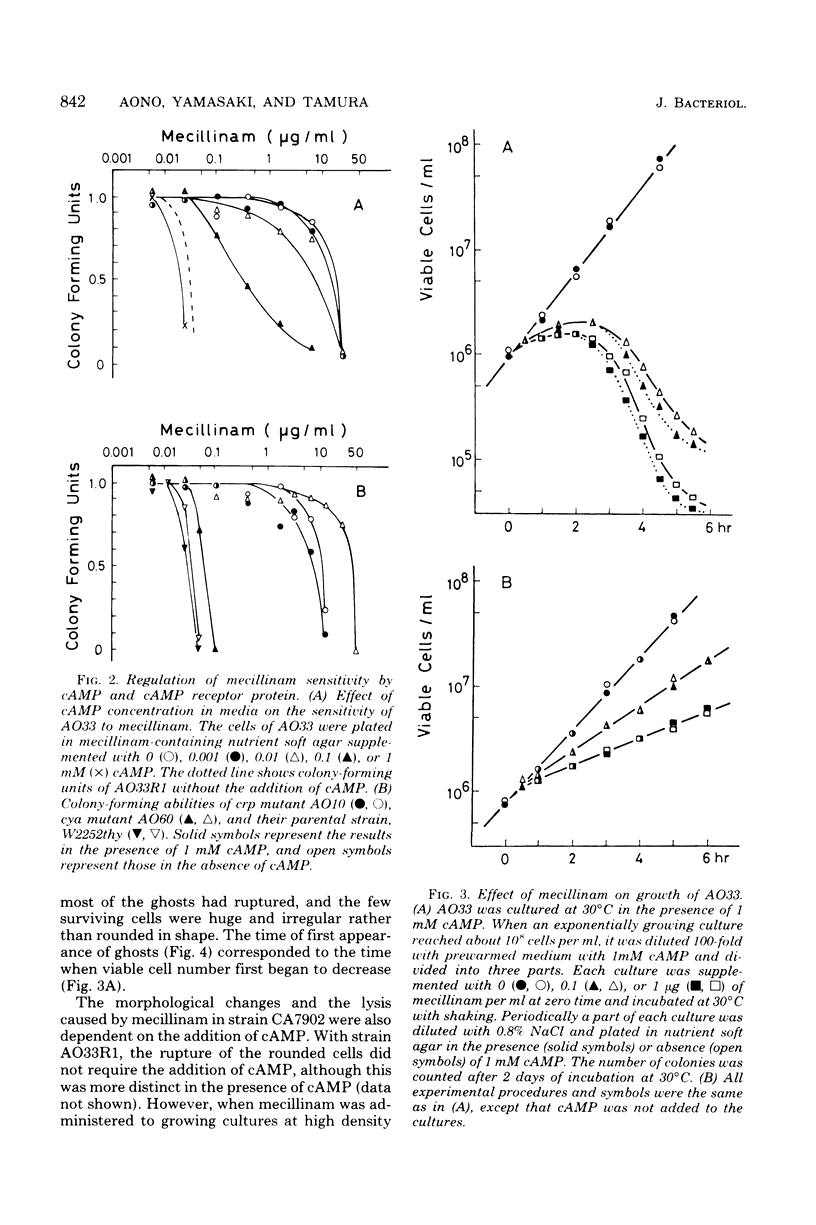
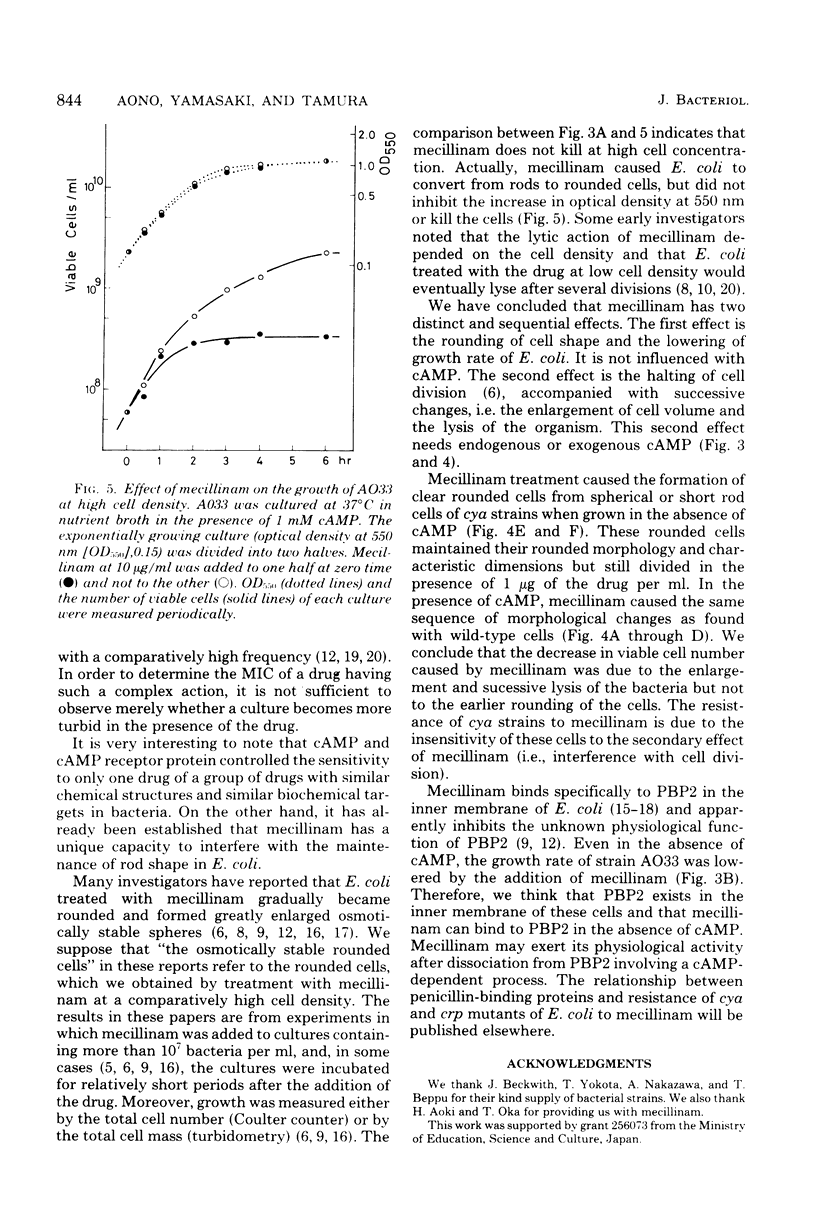
Adenylate cyclase-deficient (cya) mutants of Escherichia coli K-12 were selectively and highly resistant to mecillinam (FL1060) among several beta-lactam antibiotics in the absence of cyclic adenosine 3',5'-monophosphate (cAMP). They became sensitive to the drug in the presence of cAMP. Also, cAMP receptor protein-negative (crp) mutants, with the exception of strain 5333, were highly resistant to mecillinam in the presence and in the absence of cAMP. Mecillinam exerted two distinct and sequential effects in both cya+ strains and cya strains supplemented with cAMP: (i) rounding of cells and (ii) cessation of cell division. The first effect was accompanied by a decrease in growth rate, whereas the second effect was accompanied by enlargement and lysis of the rounded cells. The second effect of mecillinam was dependent on inoculum size and cAMP. When the cell density was above about 10(6) cells per ml, the rounded cells stopped dividing but did not lyse. In the absence of cAMP, cya strains neither stopped dividing nor lysed; they were resistant to the second, lethal effect of mecillinam.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aono R., Yamasaki M., Tamura G. Changes in composition of envelope proteins in adenylate cyclase- or cyclic AMP receptor protein-deficient mutants of Escherichia coli. J Bacteriol. 1978 Nov;136(2):812–814. doi: 10.1128/jb.136.2.812-814.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman E., Soll L., Beckwith J. Genetic characterization of mutations which affect catabolite-sensitive operons in Escherichia coli, including deletions of the gene for adenyl cyclase. J Bacteriol. 1973 Nov;116(2):582–587. doi: 10.1128/jb.116.2.582-587.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmer M., deCrombrugghe B., Pastan I., Perlman R. Cyclic AMP receptor protein of E. coli: its role in the synthesis of inducible enzymes. Proc Natl Acad Sci U S A. 1970 Jun;66(2):480–487. doi: 10.1073/pnas.66.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell E. W., Lopez R., Tomasz A. Suppression of lytic effect of beta lactams on Escherichia coli and other bacteria. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3293–3297. doi: 10.1073/pnas.73.9.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James R., Haga J. Y., Pardee A. B. Inhibition of an early event in the cell division cycle of Escherichia coli by FL1060, an amidinopenicillanic acid. J Bacteriol. 1975 Jun;122(3):1283–1292. doi: 10.1128/jb.122.3.1283-1292.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S. Properties of adenyl cyclase and cyclic adenosine 3',5'-monophosphate receptor protein-deficient mutants of Escherichia coli. J Bacteriol. 1976 Feb;125(2):545–555. doi: 10.1128/jb.125.2.545-555.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund F., Tybring L. 6 -amidinopenicillanic acids--a new group of antibiotics. Nat New Biol. 1972 Apr 5;236(66):135–137. doi: 10.1038/newbio236135a0. [DOI] [PubMed] [Google Scholar]
- Matsuhashi S., Kamiryo T., Blumberg P. M., Linnett P., Willoughby E., Strominger J. L. Mechanism of action and development of resistance to a new amidino penicillin. J Bacteriol. 1974 Feb;117(2):578–587. doi: 10.1128/jb.117.2.578-587.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C. Mecillinam, a novel penicillanic acid derivative with unusual activity against gram-negative bacteria. Antimicrob Agents Chemother. 1976 May;9(5):793–799. doi: 10.1128/aac.9.5.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. T., Burman L. FL-1060: a new penicillin with a unique mode of action. Biochem Biophys Res Commun. 1973 Apr 16;51(4):863–868. doi: 10.1016/0006-291x(73)90006-5. [DOI] [PubMed] [Google Scholar]
- Sabourin D., Beckwith J. Deletion of the Escherichia coli crp gene. J Bacteriol. 1975 Apr;122(1):338–340. doi: 10.1128/jb.122.1.338-340.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Comparison of the binding properties of two 6 beta-amidinopenicillanic acid derivatives that differ in their physiological effects on Escherichia coli. Antimicrob Agents Chemother. 1977 Jan;11(1):161–166. doi: 10.1128/aac.11.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G., Pardee A. B. Penicillin-binding proteins and cell shape in E. coli. Nature. 1975 Apr 10;254(5500):516–517. doi: 10.1038/254516a0. [DOI] [PubMed] [Google Scholar]
- Tybring L. Mecillinam (FL 1060), a 6beta-amidinopenicillanic acid derivative: in vitro evaluation. Antimicrob Agents Chemother. 1975 Sep;8(3):266–270. doi: 10.1128/aac.8.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tybring L., Melchior N. H. Mecillinam (FL 1060), a 6beta-amidinopenicillanic acid derivative: bactericidal action and synergy in vitro. Antimicrob Agents Chemother. 1975 Sep;8(3):271–276. doi: 10.1128/aac.8.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]



