Abstract
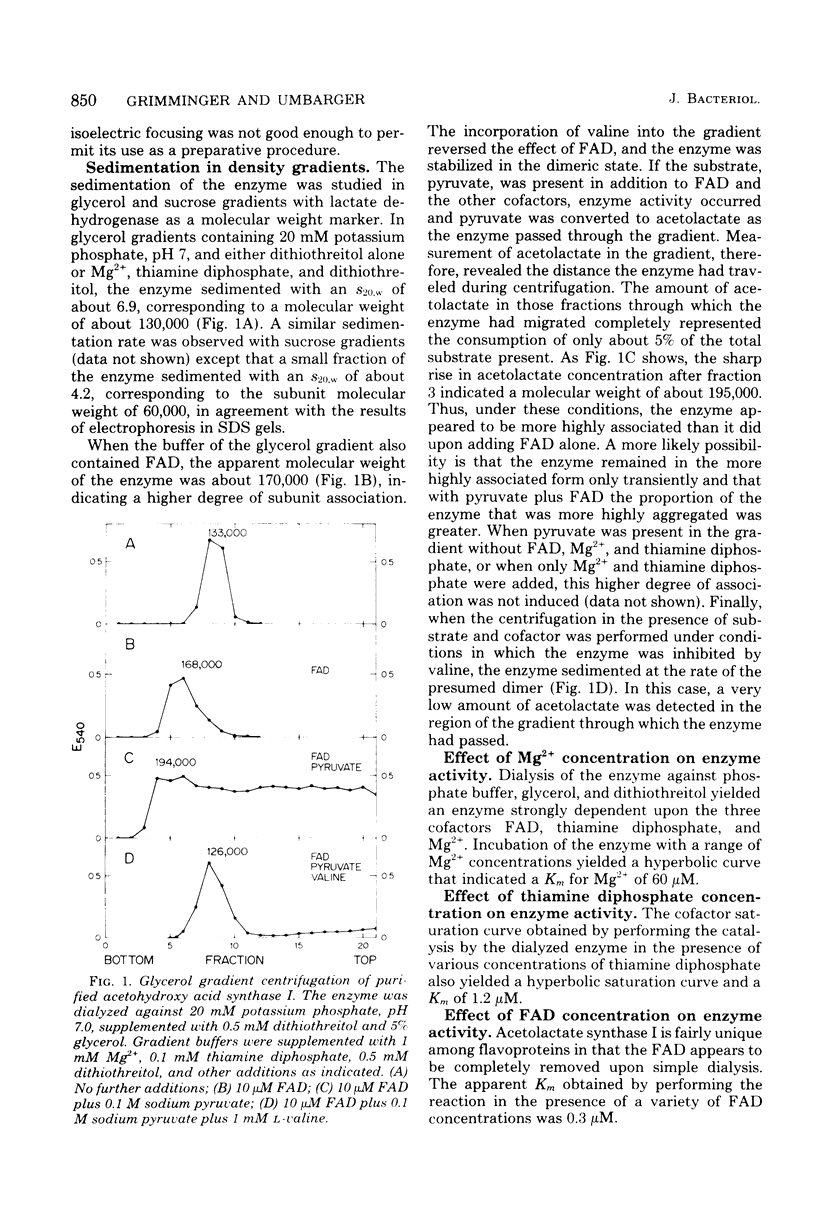
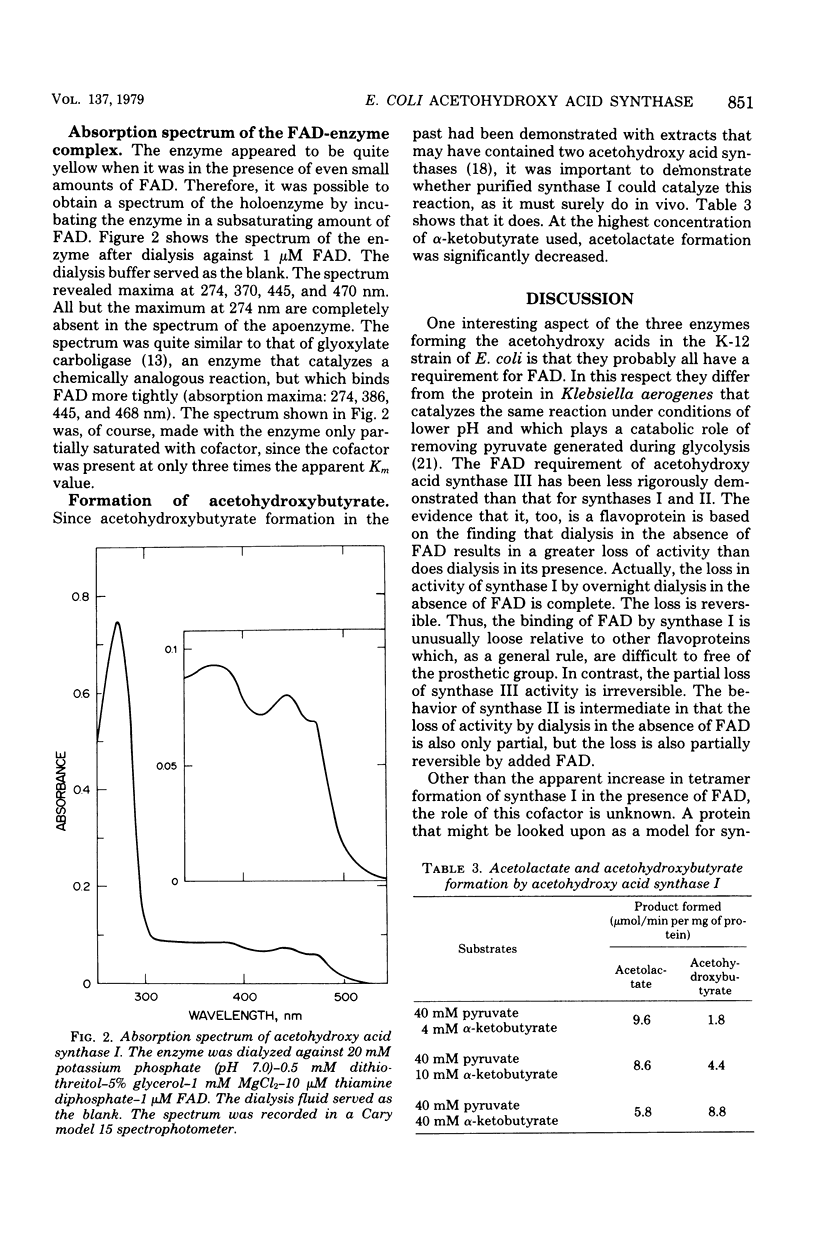
Several properties of the three acetohydroxy acid synthases of Escherichia coli have been compared in crude extracts. The three enzymes can be readily distinguished from each other. Acetohydroxy acid synthase I, the product of the ilvB gene, has been purified to near homogeneity. The purification was made possible by the fact that the enzyme was maintained in buffers of a high ionic strength or in buffers containing glycerol. Density gradient centrifugation studies indicated that the enzyme exists as a dimer of subunits of similar (60,000) molecular weight in buffers containing glycerol with or without two of the cofactors. Mg2+ and thiamine diphosphate. When flavine adenine dinucleotide was added along with Mg2+ and thiamine diphosphate, an increase in the rate of sedimentation occurred that was thought to be due to a rapid tetramer-dimer interconversion. The addition of pyruvate, the substrate, along with the three cofactors, resulted in a further increase in sedimentation rate, due presumably to an increase in the tetramer-to-dimer ratio. The addition of valine to the complete system resulted in maintenance of the enzyme in the dimeric state concomitant with inhibition of enzyme activity.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUERLE R. H., FRUENDLICH M., STORMER F. C., UMBARGER H. E. CONTROL OF ISOLEUCINE, VALINE AND LEUCINE BIOSYNTHESIS. II. ENDPRODUCT INHIBITION BY VALINE OF ACETOHYDROXY ACID SYNTHETASE IN SALMONELLA TYPHIMURIUM. Biochim Biophys Acta. 1964 Oct 23;92:142–149. [PubMed] [Google Scholar]
- Bigelis R., Umbarger H. E. Purification of yeast alpha-isopropylmalate isomerase. High ionic strength hydrophobic chromatography. J Biol Chem. 1975 Jun 10;250(11):4315–4321. [PubMed] [Google Scholar]
- Blatt J. M., Pledger W. J., Umbarger H. E. Isoleucine and valine metabolism in Escherichia coli. XX. Multiple forms of acetohydroxy acid synthetase. Biochem Biophys Res Commun. 1972 Jul 25;48(2):444–450. doi: 10.1016/s0006-291x(72)80071-8. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- De Felice M., Levinthal M. The acetohydroxy acid synthase III isoenzyme of Escherichia coli K-12: regulation of synthesis by leucine. Biochem Biophys Res Commun. 1977 Nov 7;79(1):82–87. doi: 10.1016/0006-291x(77)90063-8. [DOI] [PubMed] [Google Scholar]
- GUPTA N. K., VENNESLAND B. GLYOXYLATE CARBOLIGASE OF ESCHERICHIA COLI: A FLAVOPROTEIN. J Biol Chem. 1964 Nov;239:3787–3789. [PubMed] [Google Scholar]
- Guardiola J., De Felice M., Iaccarino M. Mutant of Escherichia coli K-12 missing acetolactate synthase activity. J Bacteriol. 1974 Oct;120(1):536–538. doi: 10.1128/jb.120.1.536-538.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardiola J., De Felice M., Lamberti A., Iaccarino M. The acetolactate synthase isoenzymes of Escherichia coli K-12. Mol Gen Genet. 1977 Nov 4;156(1):17–25. doi: 10.1007/BF00272247. [DOI] [PubMed] [Google Scholar]
- Gupta N. K., Vennesland B. Glyoxylate carboligase of Escherichia coli: some properties of the enzyme. Arch Biochem Biophys. 1966 Feb;113(2):255–264. doi: 10.1016/0003-9861(66)90185-8. [DOI] [PubMed] [Google Scholar]
- Kline E. L., Brown C. S., Coleman W. G., Jr, Umbarger H. E. Regulation of isoleucine-valine biosynthesis in an ilvDAC deletion strain of Escherichia coli K-12. Biochem Biophys Res Commun. 1974 Apr 23;57(4):1144–1151. doi: 10.1016/0006-291x(74)90816-x. [DOI] [PubMed] [Google Scholar]
- LEAVITT R. I., UMBARGER H. E. Isoleucine and valine metabolism in Escherichia coli. X. The enzymatic formation of acetohydroxybutyrate. J Biol Chem. 1961 Sep;236:2486–2491. [PubMed] [Google Scholar]
- Leary T. R., Kohlhaw G. B. -isopropylmalate synthase from Salmonella typhimurium. Analysis of the guaternary structure and its relation to function. J Biol Chem. 1972 Feb 25;247(4):1089–1095. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- O'Neill J. P., Freundlich M. Two forms of biosynthetic acetohydroxy acid synthetase in Salmonella typhimurium. Biochem Biophys Res Commun. 1972 Jul 25;48(2):437–443. doi: 10.1016/s0006-291x(72)80070-6. [DOI] [PubMed] [Google Scholar]
- Störmer F. C. The pH 6 acetolactate-forming enzyme from Aerobacter aerogenes. II. Evidence that it is not a flavoprotein. J Biol Chem. 1968 Jul 10;243(13):3740–3741. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wiginton D. A., Shive W. Control of acetohydroxy acid synthetase in Escherichia coli 9723. Biochemistry. 1978 Aug 8;17(16):3292–3297. doi: 10.1021/bi00609a018. [DOI] [PubMed] [Google Scholar]



