Abstract
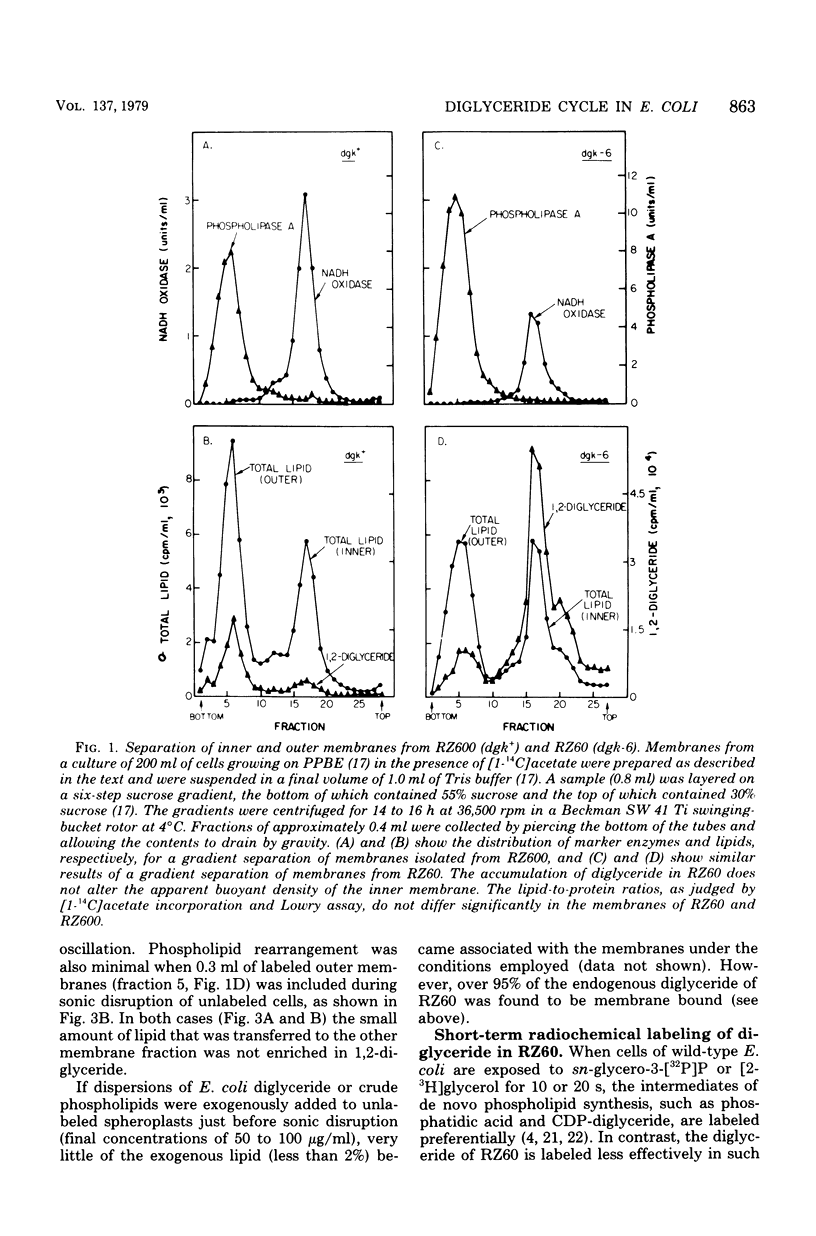
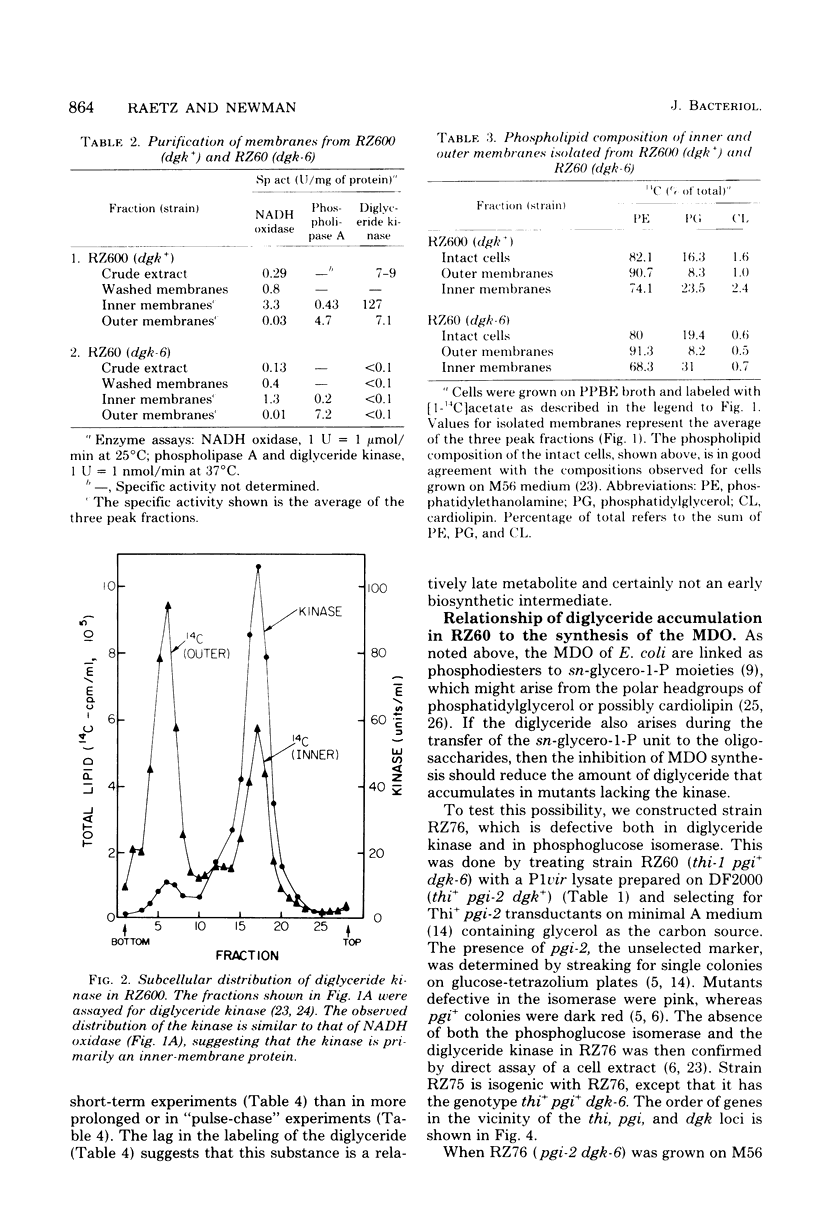
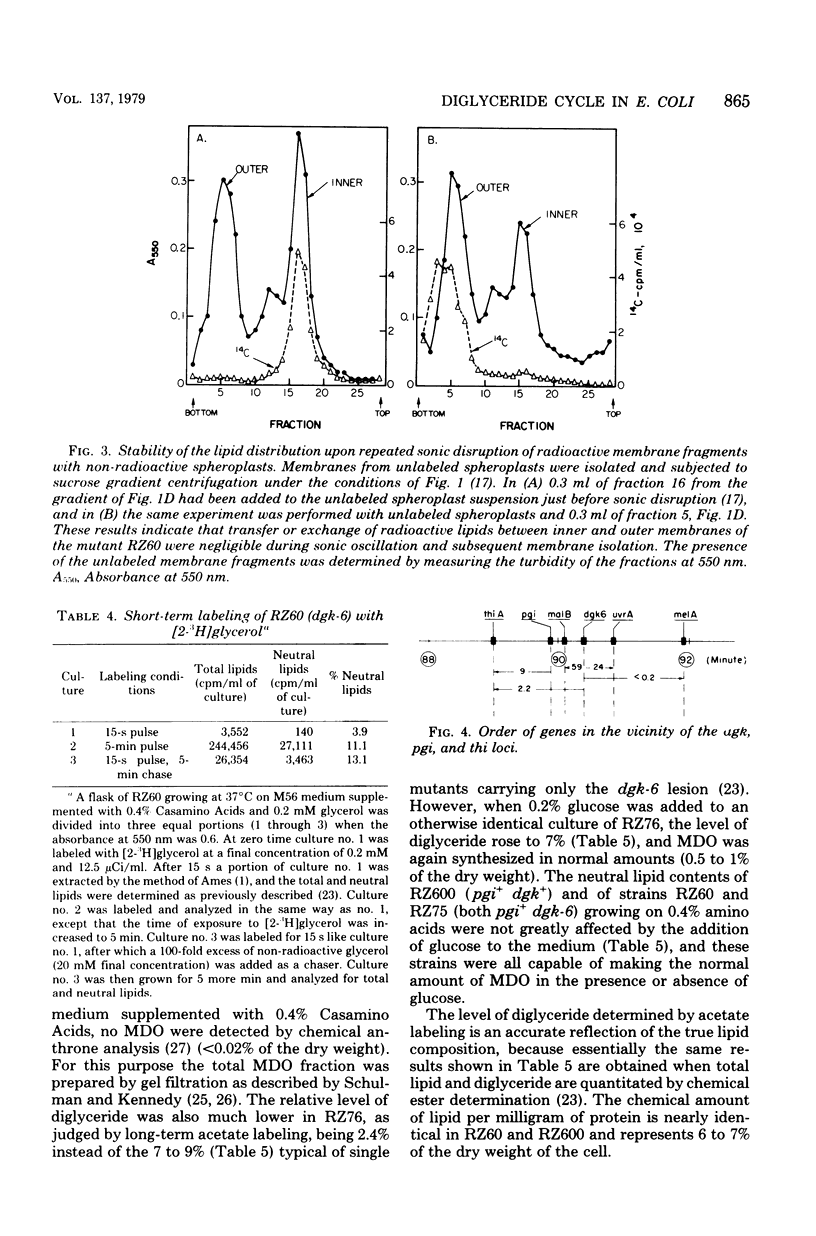
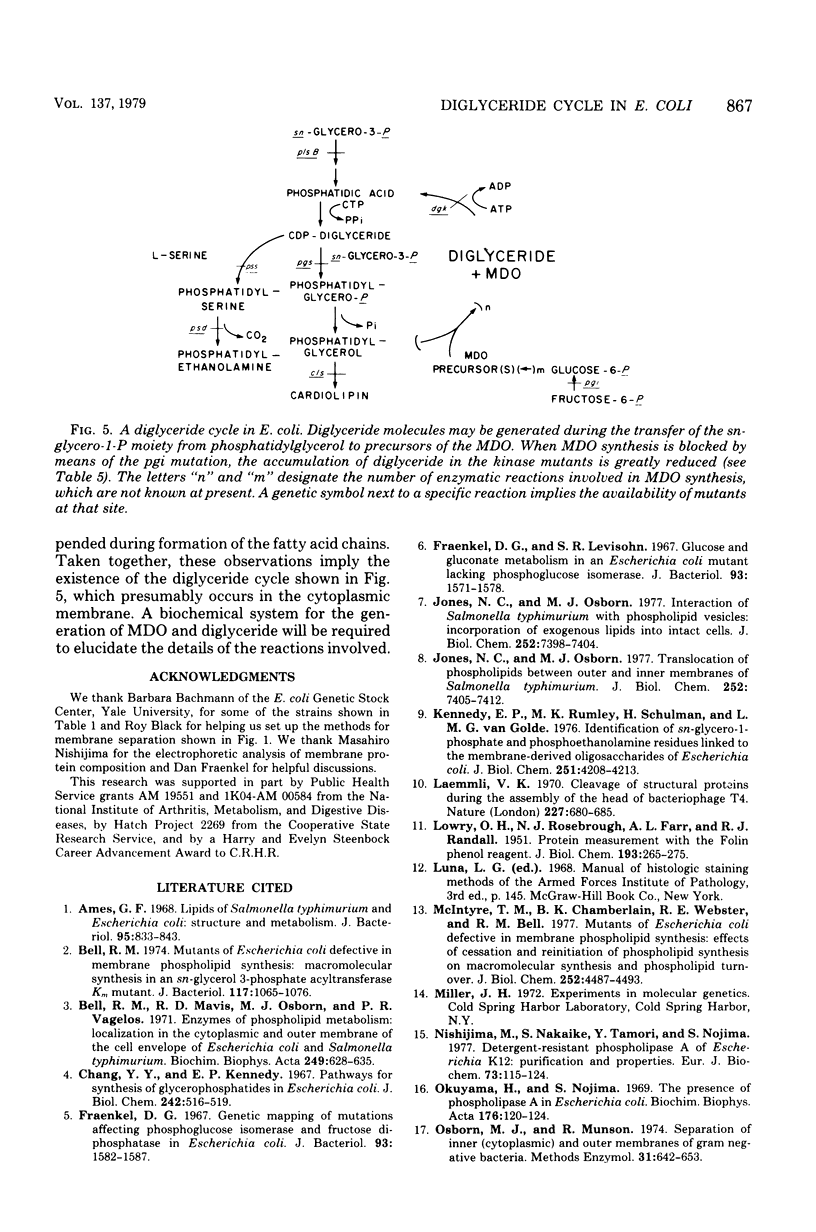
Mutants of Escherichia coli defective in diglyceride kinase contain 10 to 20 times more sn-1,2-diglyceride than normal cells. This material constitutes about 8% of the total lipid in such strains. We now report that this excess diglyceride is recovered in the particulate fraction, primarily in association with the inner, cytoplasmic membrane. The diglyceride kinase of wild-type cells was recovered in the same inner membrane fractions. The conditions employed for the preparation of the membranes did not appear to cause significant redistribution of lipids and proteins. The biochemical reactions leading to the formation of diglyceride in E. coli are not known. To determine whether diglyceride formation requires concurrent synthesis of the membrane-derived oligosaccharides (H. Schulman and E. P. Kennedy, J. Biol. Chem. 252:4250-4255, 1977), we have constructed a double mutant defective in both the kinase (dgk) and phosphoglucose isomerase (pgi). When oligosaccharide synthesis was inhibited in this organism by growing the cells on amino acids as the sole carbon source, the diglyceride was no longer present in large amounts. When glucose was also added to the medium, the pgi mutation was bypassed, oligosaccharide synthesis resumed, and diglyceride again accumulated. These findings suggest that diglyceride may arise during the transfer of the sn-glycero-1-P moiety from phosphatidylglycerol (and possibly cardiolipin) to the oligosaccharides. In wild-type cells the kinase permits the cyclical reutilization of diglyceride molecules for phospholipid biosynthesis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R. M., Mavis R. D., Osborn M. J., Vagelos P. R. Enzymes of phospholipid metabolism: localization in the cytoplasmic and outer membrane of the cell envelope of Escherichia coli and Salmonella typhimurium. Biochim Biophys Acta. 1971 Dec 3;249(2):628–635. doi: 10.1016/0005-2736(71)90144-1. [DOI] [PubMed] [Google Scholar]
- Bell R. M. Mutants of Escherichia coli defective in membrane phospholipid synthesis: macromolecular synthesis in an sn-glycerol 3-phosphate acyltransferase Km mutant. J Bacteriol. 1974 Mar;117(3):1065–1076. doi: 10.1128/jb.117.3.1065-1076.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. Y., Kennedy E. P. Pathways for the synthesis of glycerophosphatides in Escherichia coli. J Biol Chem. 1967 Feb 10;242(3):516–519. [PubMed] [Google Scholar]
- Fraenkel D. G. Genetic mapping of mutations affecting phosphoglucose isomerase and fructose diphosphatase in Escherichia coli. J Bacteriol. 1967 May;93(5):1582–1587. doi: 10.1128/jb.93.5.1582-1587.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel D. G., Levisohn S. R. Glucose and gluconate metabolism in an Escherichia coli mutant lacking phosphoglucose isomerase. J Bacteriol. 1967 May;93(5):1571–1578. doi: 10.1128/jb.93.5.1571-1578.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N. C., Osborn M. J. Interaction of Salmonella typhimurium with phospholipid vesicles. Incorporation of exogenous lipids into intact cells. J Biol Chem. 1977 Oct 25;252(20):7398–7404. [PubMed] [Google Scholar]
- Jones N. C., Osborn M. J. Translocation of phospholipids between the outer and inner membranes of Salmonella typhimurium. J Biol Chem. 1977 Oct 25;252(20):7405–7412. [PubMed] [Google Scholar]
- Kennedy E. P., Rumley M. K., Schulman H., Van Golde L. M. Identification of sn-glycero-1-phosphate and phosphoethanolamine residues linked to the membrane-derived Oligosaccharides of Escherichia coli. J Biol Chem. 1976 Jul 25;251(14):4208–4213. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McIntyre T. M., Chamberlain B. K., Webster R. E., Bell R. M. Mutants of Escherichia coli defective in membrane phospholipid synthesis. Effects of cessation and reinitiation of phospholipid synthesis on macromolecular synthesis and phospholipid turnover. J Biol Chem. 1977 Jul 10;252(13):4487–4493. [PubMed] [Google Scholar]
- Nishijima M., Nakaike S., Tamori Y., Nojima S. Detergent-resistant phospholipase A of Escherichia coli K-12. Purification and properties. Eur J Biochem. 1977 Feb 15;73(1):115–124. doi: 10.1111/j.1432-1033.1977.tb11297.x. [DOI] [PubMed] [Google Scholar]
- Okuyama H., Nojima S. The presence of phospholipase A in Escherichia coli. Biochim Biophys Acta. 1969 Jan 21;176(1):120–124. [PubMed] [Google Scholar]
- Osborn M. J., Munson R. Separation of the inner (cytoplasmic) and outer membranes of Gram-negative bacteria. Methods Enzymol. 1974;31:642–653. doi: 10.1016/0076-6879(74)31070-1. [DOI] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- PIERINGER R. A., KUNNES R. S. THE BIOSYNTHESIS OF PHOSPHATIDIC ACID AND LYSOPHOSPHATIDIC ACID BY GLYCERIDE PHOSPHOKINASE PATHWAYS IN ESCHERICHIA COLI. J Biol Chem. 1965 Jul;240:2833–2838. [PubMed] [Google Scholar]
- Proulx P. R., Van Deenen L. L. Phospholipase activities of Escherichia coli. Biochim Biophys Acta. 1967 Aug 8;144(1):171–174. doi: 10.1016/0005-2760(67)90092-6. [DOI] [PubMed] [Google Scholar]
- Raetz C. R. Enzymology, genetics, and regulation of membrane phospholipid synthesis in Escherichia coli. Microbiol Rev. 1978 Sep;42(3):614–659. doi: 10.1128/mr.42.3.614-659.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R., Kennedy E. P. Function of cytidine diphosphate-diglyceride and deoxycytidine diphosphate-diglyceride in the biogenesis of membrane lipids in Escherichia coli. J Biol Chem. 1973 Feb 10;248(3):1098–1105. [PubMed] [Google Scholar]
- Raetz C. R., Newman K. F. Neutral lipid accumulation in the membranes of Escherichia coli mutants lacking diglyceride kinase. J Biol Chem. 1978 Jun 10;253(11):3882–3887. [PubMed] [Google Scholar]
- STERN I., SHAPIRO B. A rapid and simple method for the determination of esterified fatty acids and for total fatty acids in blood. J Clin Pathol. 1953 May;6(2):158–160. doi: 10.1136/jcp.6.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider E. G., Kennedy E. P. Phosphorylation of ceramide by diglyceride kinase preparations from Escherichia coli. J Biol Chem. 1973 May 25;248(10):3739–3741. [PubMed] [Google Scholar]
- Schulman H., Kennedy E. P. Identification of UDP-glucose as an intermediate in the biosynthesis of the membrane-derived oligosaccharides of Escherichia coli. J Biol Chem. 1977 Sep 25;252(18):6299–6303. [PubMed] [Google Scholar]
- Schulman H., Kennedy E. P. Relation of turnover of membrane phospholipids to synthesis of membrane-derived oligosaccharides of Escherichia coli. J Biol Chem. 1977 Jun 25;252(12):4250–4255. [PubMed] [Google Scholar]
- Weissbach H., Thomas E., Kaback H. R. Studies on the metabolism of ATP by isolated bacterial membranes: formation and metabolism of membrane-bound phosphatidic acid. Arch Biochem Biophys. 1971 Nov;147(1):249–254. doi: 10.1016/0003-9861(71)90332-8. [DOI] [PubMed] [Google Scholar]
- White D. A., Albright F. R., Lennarz W. J., Schnaitman C. A. Distribution of phospholipid-synthesizing enzymes in the wall and membrane subfractions of the envelope of Escherichia coli. Biochim Biophys Acta. 1971 Dec 3;249(2):636–642. doi: 10.1016/0005-2736(71)90145-3. [DOI] [PubMed] [Google Scholar]
- van Golde L. M. Metabolism of membrane phospholipids and its relation to a novel class of oligosaccharides in Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1368–1372. doi: 10.1073/pnas.70.5.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]



