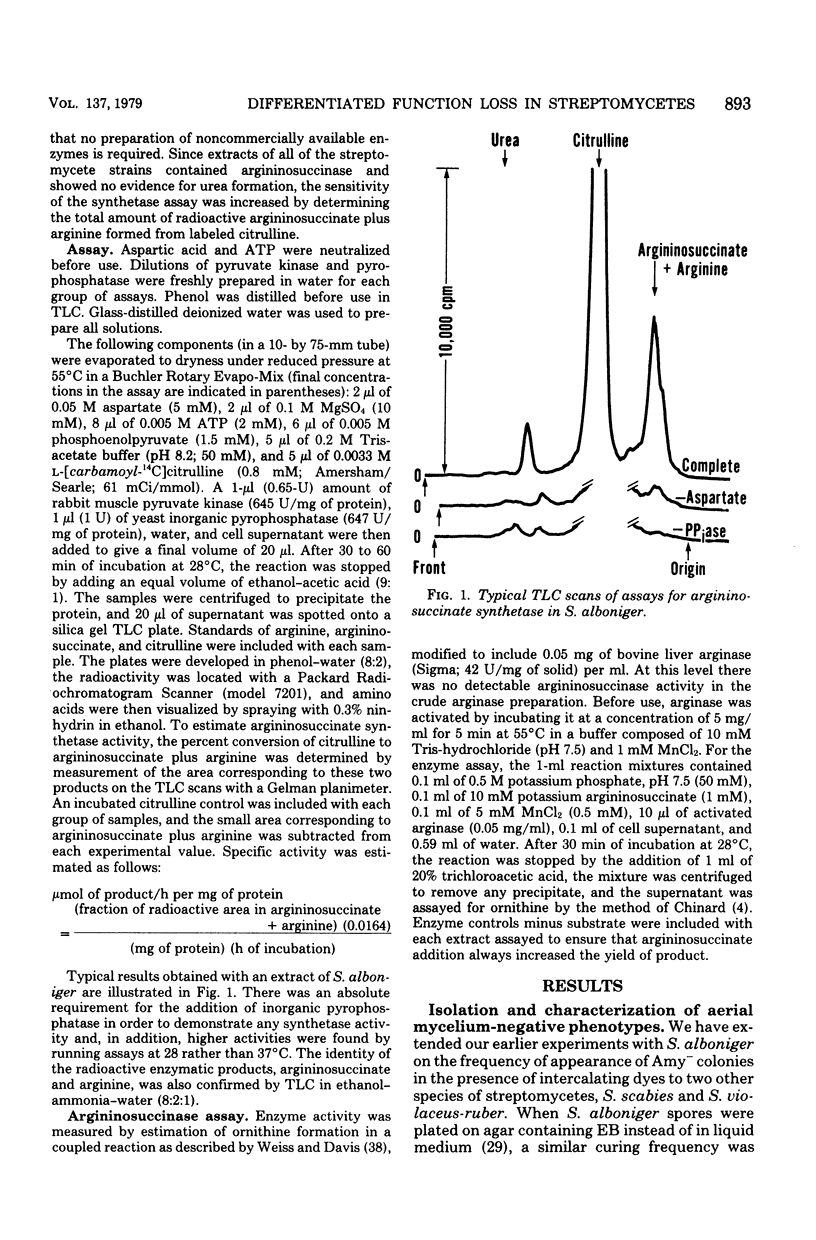
Abstract
Germination and outgrowth of spores of Streptomyces alboniger, Streptomyces scabies, and Streptomyces violaceus-ruber in the presence of intercalating dyes resulted in a high frequency (2 to 20%) of occurrence of aerial mycelium-negative (Amy-) isolates. Coincident with the appearance of the Amy- trait was the loss of several differentiated functions, including the characteristic pigments and earthy odor of the wild types. All S. alboniger, 27% of S. scabies, and 39% of the S. violaceus-ruber Amy- isolates were arginine auxotrophs. The missing enzyme step was identified as argininosuccinate synthetase by using a sensitive microassay for estimation of enzyme activity. The remainder of the S. scabies and S. violaceus-ruber isolates were prototrophs. In addition, S. alboniger Amy- isolates failed to produce or respond to the stimulator of aerial mycelium formation isolated from the wild type. The Amy- isolates did not revert to either Amy+ of Arg+. The lack of any detectable reversion, coupled with the high frequency of curing, supports the idea that a deletion of genetic material, possibly a plasmid, has occurred.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akagawa H., Okanishi M., Umezawa H. A plasmid involved in chloramphenicol production in Streptomyces venezuelae: evidence from genetic mapping. J Gen Microbiol. 1975 Oct;90(2):336–346. doi: 10.1099/00221287-90-2-336. [DOI] [PubMed] [Google Scholar]
- CHINARD F. P. Photometric estimation of proline and ornithine. J Biol Chem. 1952 Nov;199(1):91–95. [PubMed] [Google Scholar]
- Delavier-Klutchko C., Ebersolt C., Daniel C. Exigence en arginine et lysine de Ustilago cynodontis 4001, forme levure. I. --Croissance en présence et en absence de lysine. Ann Microbiol (Paris) 1976 Apr;127(3):353–366. [PubMed] [Google Scholar]
- Durieu-Trautmann O., Tavlitzki J. Reversible and permanent effects of the carbon sources and various antibiotics on the morphology and metabolic properties of Ustilago cynodontis cells. J Cell Biol. 1975 Jul;66(1):102–113. doi: 10.1083/jcb.66.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREGORY K. F., HUANG J. C. TYROSINASE INHERITANCE IN STREPTOMYCES SCABIES. I. GENETIC RECOMBINATION. J Bacteriol. 1964 Jun;87:1281–1286. doi: 10.1128/jb.87.6.1281-1286.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREGORY K. F., HUANG J. C. TYROSINASE INHERITANCE IN STREPTOMYCES SCABIES. II. INDUCTION OF TYROSINASE DEFICIENCY BY ACRIDINE DYES. J Bacteriol. 1964 Jun;87:1287–1294. doi: 10.1128/jb.87.6.1287-1294.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HICKEY R. J., TRESNER H. D. A cobalt-containing medium for sporulation of Streptomyces species. J Bacteriol. 1952 Dec;64(6):891–892. doi: 10.1128/jb.64.6.891-892.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIROTA Y., LIJIMA T. Acriflavine as an effective agent for eliminating F-factor in Escherichia coli K-12. Nature. 1957 Sep 28;180(4587):655–656. doi: 10.1038/180655a0. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Chater K. F., Dowding J. E., Vivian A. Advances in Streptomyces coelicolor genetics. Bacteriol Rev. 1973 Sep;37(3):371–405. doi: 10.1128/br.37.3.371-405.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood D. A. Genetic analysis and genome structure in Streptomyces coelicolor. Bacteriol Rev. 1967 Dec;31(4):373–403. doi: 10.1128/br.31.4.373-403.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood D. A., Wright H. M. A plasmid of Streptomyces coelicolor carrying a chromosomal locus and its inter-specific transfer. J Gen Microbiol. 1973 Dec;79(2):331–342. doi: 10.1099/00221287-79-2-331. [DOI] [PubMed] [Google Scholar]
- Kalakoutskii L. V., Agre N. S. Comparative aspects of development and differentiation in actinomycetes. Bacteriol Rev. 1976 Jun;40(2):469–524. doi: 10.1128/br.40.2.469-524.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby R., Wright L. F., Hopwood D. A. Plasmid-determined antibiotic synthesis and resistance in Streptomyces coelicolor. Nature. 1975 Mar 20;254(5497):265–267. doi: 10.1038/254265a0. [DOI] [PubMed] [Google Scholar]
- Kleckner N. Translocatable elements in procaryotes. Cell. 1977 May;11(1):11–23. doi: 10.1016/0092-8674(77)90313-0. [DOI] [PubMed] [Google Scholar]
- Kähler R., Noack D. Action of acridine orange and ethidium bromide on growth and antibiotic activity of Streptomyces hygroscopicus JA 6599. Z Allg Mikrobiol. 1974;14(6):529–533. doi: 10.1002/jobm.3630140610. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Malik V. S. Preparative method for the isolation of super-coiled DNA from a chloramphenicol-producing streptomycete. J Antibiot (Tokyo) 1977 Oct;30(10):897–899. doi: 10.7164/antibiotics.30.897. [DOI] [PubMed] [Google Scholar]
- Morse M L, Lederberg E M, Lederberg J. Transduction in Escherichia Coli K-12. Genetics. 1956 Jan;41(1):142–156. doi: 10.1093/genetics/41.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P. Extrachromosomal inheritance in bacteria. Bacteriol Rev. 1969 Jun;33(2):210–263. doi: 10.1128/br.33.2.210-263.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okanishi M., Ita T., Umezawa H. Possible control of formation of aerial mycelium and antibiotic production in Streptomyces by episomic factors. J Antibiot (Tokyo) 1970 Jan;23(1):45–47. doi: 10.7164/antibiotics.23.45. [DOI] [PubMed] [Google Scholar]
- Rao M. M., Rebello P. F., Pogell B. M. Biosynthesis of puromycin in Streptomyces alboniger. Enzymatic methylation of O-demethylpuromycin. J Biol Chem. 1969 Jan 10;244(1):112–118. [PubMed] [Google Scholar]
- Redshaw P. A., McCann P. A., Sankaran L., Pogell B. M. Control of differentiation in streptomycetes: involvement of extrachromosomal deoxyribonucleic acid and glucose repression in aerial mycelia development. J Bacteriol. 1976 Feb;125(2):698–705. doi: 10.1128/jb.125.2.698-705.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J. R. Frameshift mutations. Annu Rev Genet. 1974;8:319–346. doi: 10.1146/annurev.ge.08.120174.001535. [DOI] [PubMed] [Google Scholar]
- Sankaran L., Pogell B. M. Biosynthesis of puromycin in Streptomyces alboniger: regulation and properties of O-demethylpuromycin O-methyltransferase. Antimicrob Agents Chemother. 1975 Dec;8(6):721–732. doi: 10.1128/aac.8.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrempf H., Bujard H., Hopwood D. A., Goebel W. Isolation of covalently closed circular deoxyribonucleic acid from Streptomyces coelicolor A3(2). J Bacteriol. 1975 Feb;121(2):416–421. doi: 10.1128/jb.121.2.416-421.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrempf H., Goebel W. Characterization of a plasmid from Streptomyces coelicolor A3(2). J Bacteriol. 1977 Jul;131(1):251–258. doi: 10.1128/jb.131.1.251-258.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sermonti G., Petris A., Micheli M., Lanfaloni L. Chloramphenicol resistance in Streptomyces coelicolor A3(2): possible involvement of a transposable element. Mol Gen Genet. 1978 Aug 4;164(1):99–103. doi: 10.1007/BF00267604. [DOI] [PubMed] [Google Scholar]
- Shaw P. D., Piwowarski J. Effects of ethidium bromide and acriflavine on streptomycin production by Streptomyces bikiniensis. J Antibiot (Tokyo) 1977 May;30(5):404–408. doi: 10.7164/antibiotics.30.404. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Revised linkage map of Escherichia coli. Bacteriol Rev. 1967 Dec;31(4):332–353. doi: 10.1128/br.31.4.332-353.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. L., Davis R. H. Intracellular localization of enzymes of arginine metabolism in Neurospora. J Biol Chem. 1973 Aug 10;248(15):5403–5408. [PubMed] [Google Scholar]
- Wright L. F., Hopwood D. A. Identification of the antibiotic determined by the SCP1 plasmid of Streptomyces coelicolor A3(2). J Gen Microbiol. 1976 Jul;95(1):96–106. doi: 10.1099/00221287-95-1-96. [DOI] [PubMed] [Google Scholar]





