Abstract
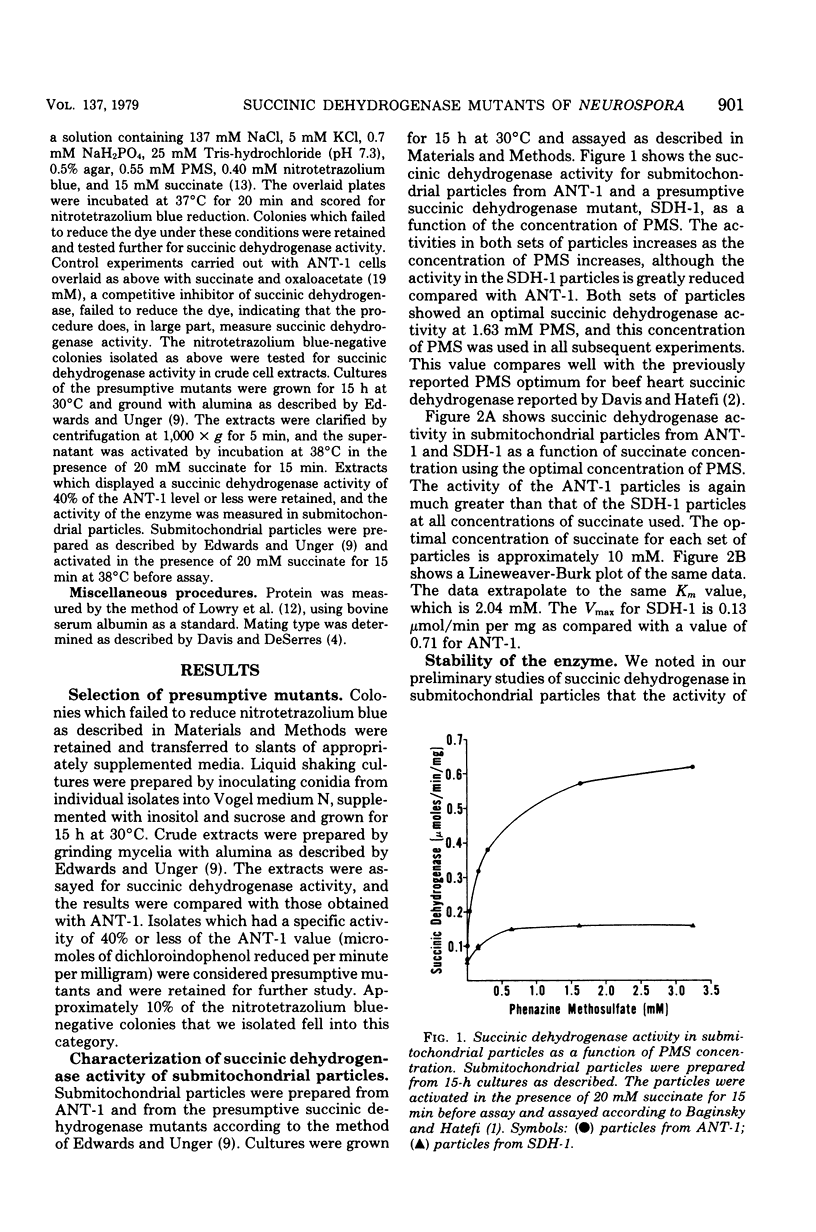
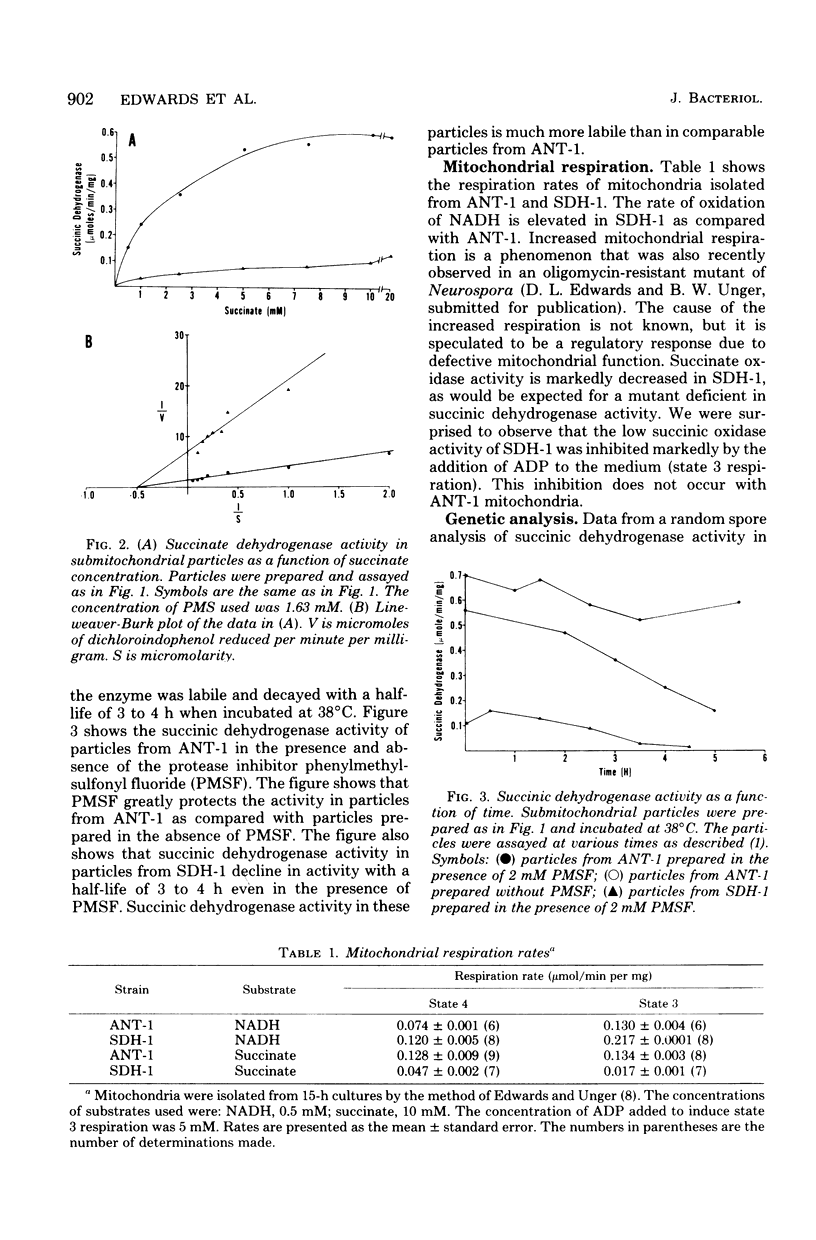
A method is described which permits the selection of mutants of Neurospora crassa that are deficient in succinic dehydrogenase activity. The method relies on the observation that succinic dehydrogenase-deficient strains fail to reduce the dye nitrotetrazolium blue when overlaid with the dye in the presence of succinate and phenazine methosulfate. Wild-type colonies reduced the dye and turned blue, whereas mutant colonies remained colorless. In this communication we present studies of a mutant, SDH-1, isolated by this method. The mutant had 18% of the succinic dehydrogenase activity of the parent strain used in the mutation experiments as determined from the ratio of Vmax activities obtained from Lineweaver-Burk plots. The SDH-1 mutant segregated in a Mendelian manner when back-crossed to its parent strain. Succinate oxidase activity in SDH-1 was low and was markedly inhibited by adenosine 5'-diphosphate. The succinate oxidase activity of the parent strain was high and was not affected by the presence of adenosine 5'-diphosphate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baginsky M. L., Hatefi Y. Reconstitution of succinate-coenzyme Q reductase (complex II) and succinate oxidase activities by a highly purified, reactivated succinate dehydrogenase. J Biol Chem. 1969 Oct 10;244(19):5313–5319. [PubMed] [Google Scholar]
- Davis K. A., Hatefi Y., Crawford I. P., Baltscheffsky H. Purification, molecular properties, and amino acid composition of the subunits of Rhodospirillum rubrum succinate dehydrogenase. Arch Biochem Biophys. 1977 Apr 30;180(2):459–464. doi: 10.1016/0003-9861(77)90060-1. [DOI] [PubMed] [Google Scholar]
- Davis K. A., Hatefi Y. Succinate dehydrogenase. I. Purification, molecular properties, and substructure. Biochemistry. 1971 Jun 22;10(13):2509–2516. doi: 10.1021/bi00789a014. [DOI] [PubMed] [Google Scholar]
- Edwards D. L., Kwiecinski F., Horstmann J. Selection of respiratory mutants of Neurospora crassa. J Bacteriol. 1973 Apr;114(1):164–168. doi: 10.1128/jb.114.1.164-168.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D. L., Rsenberg E., Maroney P. A. Induction of cyanide-insensitive respiration in Neurospora crassa. J Biol Chem. 1974 Jun 10;249(11):3551–3556. [PubMed] [Google Scholar]
- Edwards D. L., Unger B. W. Cyanide- and hydroxamate-resistant respiration in Neurospora crassa. J Bacteriol. 1978 Mar;133(3):1130–1134. doi: 10.1128/jb.133.3.1130-1134.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D. L., Unger B. W. Nuclear mutations conferring oligomycin resistance in Neurospora crassa. J Biol Chem. 1978 Jun 25;253(12):4254–4258. [PubMed] [Google Scholar]
- Gutman M., Kearney E. B., Singer T. P. Control of succinate dehydrogenase in mitochondria. Biochemistry. 1971 Dec 7;10(25):4763–4770. doi: 10.1021/bi00801a025. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Soderberg K. L., Ditta G. S., Scheffler I. E. Mammalian cells with defective mitochondrial functions: a Chinese hamster mutant cell line lacking succinate dehydrogenase activity. Cell. 1977 Apr;10(4):697–702. doi: 10.1016/0092-8674(77)90103-9. [DOI] [PubMed] [Google Scholar]
- Stuchell R. N., Weinstein B. I., Beattie D. S. Effects of ethidium bromide on the respiratory chain and oligomycin-sensitive adenosine triphosphatase in purified mitochondria from the cellular slime mold Dicyostelium discoideum. J Biol Chem. 1975 Jan 25;250(2):570–576. [PubMed] [Google Scholar]


