Abstract
Biodistribution studies of a water-soluble radioactive metallofullerene compound have been conducted using BALB/c mice. To this end, a sample containing Hox@C82 (x = 1, 2) was purified and derivatized to prepare the water-soluble metallofullerol, Hox@C82(OH)y. This metallofullerol was then neutron-activated (165Ho[n,γ]166Ho) to prepare the 166Hox@C82(OH)y analog as a radiotracer, which was monitored, after intravenous administration, for up to 48 hours by using dissection radioanalysis, and its biodistribution was compared with a control compound, Na2[166Ho(DTPA)(H2O)]. Results showed selective localization of the 166Hox@C82(OH)y tracer in the liver but with slow clearance, as well as uptake by bone without clearance. In contrast, excretion of the control compound was nearly quantitative after 1 hour. The fate of 166Ho was also explored by a metabolism study of 166Hox@C82(OH)y in Fischer rats. Results indicated 20% excretion of intact 166Hox@C82(OH)y within 5 days. The present findings demonstrate the feasibility of using water-solubilized metallofullerene radiotracers to monitor the fate of fullerene-based materials in animals, and suggest that water-solubilized fullerene materials, in general, may be useful components in drug design.
Since the discovery of fullerenes in 1985 (1) and the subsequent incorporation of metal atoms within their carbon cage (2), intense interest has focused on these unique molecules. Research efforts have greatly extended our knowledge of the chemical and biological properties of empty fullerenes (3; for review, see ref. 4), but comparatively little is known about endohedral metallofullerenes because of their low production yields, low solution solubilities, difficult purification, and air sensitivity (5–9). However, advances in HPLC purification procedures (5–9) and chemical derivatization (10–15) of fullerenes have recently expanded research possibilities into applications for metallofullerenes when relatively small amounts of material are required.
One potential application for metallofullerenes is in the field of nuclear medicine. Current radiopharmaceuticals employ small quantities (nanograms to milligrams) of drugs containing specially chelated radioisotopes of metals for imaging or therapeutic applications. The chelating ligands prevent direct binding of the toxic metal ions with serum components and tissue by providing a thermodynamically stable molecular environment. A major concern with these drugs, however, is their in vivo kinetic instability, which can allow the release of small amounts of toxic radiometals (16). It is envisioned, therefore, that metallofullerenes could provide a unique alternative to chelating compounds because of their resistance to metabolism and their high kinetic stability. Additionally, the large carbon-based surface area of fullerenes and metallofullerenes (≈200 Å2) facilitates the development of tissue-targeting compounds by using established metallofullerene derivative chemistry (10–15). Thus, the metallofullerenes may be useful as a new, more stable alternative for transporting radiometals in vivo.
Empty fullerenes have low cytotoxicity both in vitro (17–22) and in vivo (17, 23–26), despite one report of a photosensitizing C60 derivative (24). In addition, empty fullerenes and their derivatives have been tested for their biodistribution, and some C60 derivatives have shown promise as anti-HIV (27–34) and anticancer (35–37) agents and as an agent to reduce reactive oxygen species (20, 21). Dugan et al. (17, 38) have also demonstrated the usefulness of fullerene derivatives as biological free-radical scavengers in vivo. Research involving the biodistribution of fullerenes in vivo is limited to studies on C60 and La@C82 suspensions (23, 25), and to three water-soluble C60 derivatives (26, 30, 39). All of these studies indicate rapid localization and long-term residency in the liver (<1% clearance), and one of the studies demonstrated that fullerenes are not metabolized rapidly in vivo (39). To help optimize the biocompatibility of metallofullerenes in vivo, a published procedure for the synthesis of C60(OH)y (y = 16) was adapted to prepare a water-soluble Hox@C82(OH)y metallofullerol from Hox@C82 (see below). This method was chosen because (i) similar polyhydroxylated species, called stealth liposomes, have reduced recognition and uptake by the body’s reticuloendotholial system (40, 41) and (ii) the reaction is straightforward and proceeds in high yield by using only milligrams of metallofullerene material.
Holmium metallofullerene was chosen because of the natural monoisotopic abundance of 165Ho, the proven utility of 166Ho in therapeutic nuclear medicine (166Ho: t1/2 = 26.8 h, Eβmax = 1.8 MeV) (42–44), and the high cross section of 165Ho, which undergoes neutron capture to produce radioactive 166Ho (σ = 61.2 barn). In addition, the properties of Hox@C82 (x = 1, 2) are now relatively well understood, having been characterized previously by laser desorption–time-of-flight mass spectrometry (LD-TOF MS), EPR spectroscopy (45, 46), purification techniques (47), and electrochemical studies (48).
As a prelude to the development of a metallofullerene-based radiotracer, C60 was found to remain structurally intact (99%) in a neutron flux of 1.5 × 1012 neutron⋅cm−2⋅sec−1 by Ehrhardt and coworkers (49). Thereafter, neutron-activation studies of 165Hox@C82 (50, 51) and 165Hox@C82(OH)y (50) [x = 1, 2¶; y = ≈16 (52)‖] were performed to optimize production and survivability of their radioactive analogs.
After the synthesis, purification, and neutron-activation steps described above, the qualitative biodistribution of 166Hox@C82(OH)y was tested in two Sprague–Dawley rats by using a γ-camera to monitor the 166Ho metallofullerol radiotracer over 48 hours (50). This preliminary study suggested that metallofullerols had the unusual property of a long residency time in the blood pool. For this reason, biodistribution and metabolism studies of a metallofullerol compound were conducted by using 166Hox@C82(OH)y in BALB/c mice and in Fischer rats.
MATERIALS AND METHODS
Synthesis and Purification of Hox@C82 (x = 1, 2).
Holmium-doped graphite electrodes were prepared by using a previously reported method (51). Graphite rods of 0.625 cm (0.25 inch) diameter, 15 cm (6 inch) length and 40% porosity (Poco Graphite, Decatur, TX) were placed in an evacuated chamber (<1 torr; 1 torr = 133 Pa) for 10 mins. Afterward, a saturated ethanolic solution of Ho(NO3)3⋅xH2O (x = 5–6; Aesar) was injected into the evacuated chamber, and the rods were submerged for 30 min. The rods were then dried in air and evacuated at room temperature for 1 h before heating them to 500°C at a rate of 1°C min−1. The rods were then maintained at 900°C under vacuum for 5 h to convert the metal salt to Ho2O3. The resultant holmium-impregnated graphite rods indicated mass gains commensurate with a Ho:C molar ratio of 0.75:100 assuming total conversion of the holmium nitrate to Ho2O3.
Endohedral holmium metallofullerenes were synthesized in a custom-designed carbon-arc furnace at 150 torr He (J.M.A., unpublished data). Typically, three metal-impregnated graphite rods were vaporized by an electric arc during a single fullerene generation procedure. Metallofullerenes were concentrated in the resultant soot by subliming most of the empty fullerenes onto a sublimation target at 500°C for 4 h in situ. Afterward, a second vacuum sublimation was performed from 500°C to 750°C while heating at a rate of 0.5°C min−1 to collect a highly enriched metallofullerene fraction. The enriched, sublimed material was collected under anaerobic conditions and Soxhlet-extracted under argon with degassed CS2 for 8 h. For sample storage, the CS2 Soxhlet extract was kept frozen at 77 K under nitrogen atmosphere and in the dark. When samples were needed, the solution was thawed and the solvent was evaporated to dryness under a stream of argon.
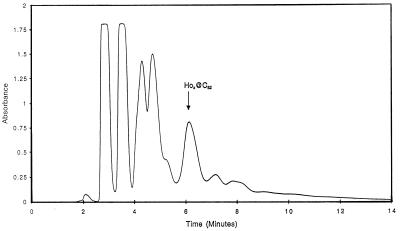
The solid, metallofullerene-enriched material was dissolved in distilled, degassed o-xylene (≈2 mg⋅ml−1), and filtered through a 0.22 μm Acrodisc nylon filter (Fisher Scientific). Chromatographic separation of Hox@C82 from empty fullerene mixtures was performed under anaerobic conditions on a Hitachi L-6200A Intelligent Pump HPLC system coupled to a Hitachi L-6000 HPLC pump and two 2-liter Kontes Ultra-Ware solvent delivery tanks. Peak detection was performed by using a Hitachi model L-3000 UV-Vis photodiode array detector (200–520 nm). Data acquisition and analysis was accomplished with Hitachi Model D-6000 chromatography data system software. All injections were 2 ml and were chromatographed under anaerobic conditions using freshly distilled, degassed o-xylene (8 ml/min, Aldrich) as the mobile phase on a Buckyprep HPLC column (10 mm × 250 mm; Nacalai Tesque, Kyoto). All peaks in the resulting chromatograph (Fig. 1) were collected and analyzed by LD-TOF MS to determine their chemical compositions.
Figure 1.
HPLC Separation of o-xylene-soluble high-temperature holmium metallofullerene sublimate from a Buckyprep column under anaerobic conditions (flow rate of 8 ml⋅min−1, retention time (Hox@C82) = 5.8–6.4 min, 305 nm).
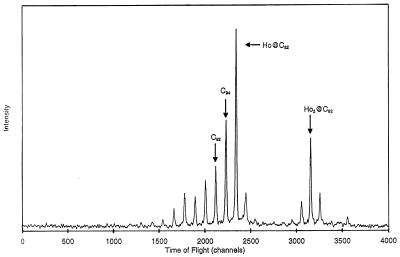
After LD-TOF MS analysis (Fig. 2), the metallofullerene-containing fraction (Fig. 1) was collected from several injections and stored in a liquid N2-cooled Schlenck flask under N2 atmosphere. When needed, the samples from pooled runs were thawed, and the solvent was removed in vacuo at ≈50°C. The solid was then weighed and redissolved in degassed o-xylene (≈2 mg⋅ml−1) at a standard concentration and refrozen until needed. The holmium content in the metallofullerene solutions was determined by using inductively coupled plasma atomic emission spectroscopy (ICP-AE; see below).
Figure 2.
LD-TOF MS Data of the HPLC-purified fraction containing Hox@C82 (x = 1, 2).
LD-TOF MS.
Mass spectrometry was performed by using LD-TOF MS at TDA Research. Samples were deposited by solution evaporation onto a 1-cm (diameter) steel disc and evacuated to 10−7 torr before analysis. Sample desorption and ionization was achieved by using 355 nm light from a Nd-YAG laser third harmonic. Detection was achieved by using a microchannel plate ion multiplier detector.
ICP-AE.
For metals analysis, samples were digested in 50 ml of concentrated HNO3 for 1 h in air. The sample was then evaporated to dryness and diluted to a known volume with ICP eluent solution [2% (wt/vol) HNO3 and 0.1% (wt/vol) nonionic surfactant (Micron, Fisher Scientific) in deionized water]. Measurements were obtained by using a Perkin–Elmer Plasma 400 ICP-AE spectrometer with data collection software. Emission lines for all standards were determined four times, whereas the unknowns were determined eight times. After data collection, the mean of each set of readings was calculated in comparison with a blank.
Holmium ICP-AE standards were made from a 10 mg⋅ml−1 ICP standard solution (Aldrich). Standard dilutions of 10, 1, 0.1, and 0.01 mg⋅ml−1 Ho3+ were freshly prepared immediately before analysis. The standards and unknowns were then evaluated for the intensity of the holmium emission line at 345.6 nm. The lower detection limit of the instrument for Ho3+ was 5.7 × 10−3 μg⋅ml−1.
Neutron-Activation Analysis (NAA) and Radioactivity Measurements.
Neutron irradiation analysis of the unsubstituted holmium metallofullerenes was performed by evaporating a known volume of Ho-metallofullerene stock solutions in an envelope made of reactor-grade aluminum foil. The envelope was packaged inside a graphite “rabbit” pneumatic tube vessel. The sample was then irradiated for 1 min in pneumatic tube no. 1 at the Oak Ridge high flux isotope reactor neutron activation analysis facility. The neutron flux employed was 4.3 × 1014 neutron⋅cm−2⋅sec−1 with a thermal neutron component of 99.7%.
Approximately 15 hours after irradiation, the sample envelope was removed from its graphite rabbit, and the 80.5 keV (6.2% abundance) γ-emission of 166Ho was measured by using a calibrated, high-purity germanium detector of 50 cm3 (EG & G, Oak Ridge, TN). Analysis of the data was achieved by using a PC-based multichannel analyzer (Canberra Industries, Meridian, CT) Samples were counted for 300 s at a calibrated position 10 cm from the detector.
The 166Ho number (dps) at the end of irradiation was then calculated after appropriate corrections were made for the γ-ray intensity (0.062), detector efficiency (7.8 × 10−3), and nuclear decay (t1/2 = 26.8 h). The 165Ho number was calculated from 166Ho activity by using the values for the reaction cross section (61.2 barn) and a saturation factor of 4.44 × 10−4 (for 1 min of irradiation).
A well-type ionization chamber was used for gross activity measurements. For biodistribution studies, an automatic γ-counter equipped with a NaI(Tl) detector (Minaxi Auto-Gamma 5000, Packard) was employed.
Preparation and Purification of Hox@C82(OH)y.
Hox@C82(OH)y was prepared via a synthesis that was a slight modification of that used for the polyhydroxylation of C60 (53). Derivatization was performed by preparing a greenish-gold, degassed o-xylene solution (≈100 ml) containing a known mass of the postchromatography metallofullerene solid described above. This solution was then stirred for 2 h under argon atmosphere with a solution containing 5 drops of tetrabutylammonium hydroxide (TBAOH, 40% in H2O, Aldrich) as a phase-transfer catalyst and 5 ml of a concentrated aqueous KOH solution. After stirring for 10 min, a thick, brown film was observed on the walls of the flask. The reaction mixture was then allowed to stir for an additional 10 h in air. Afterward, the solvents were removed in vacuo at 50°C.
After solvent removal, the reaction mixture was stirred with 20 ml of H2O/CH3OH (95%:5%) for 8 h, during which period most of the solid slowly dissolved. The supernatant was then vacuum-filtered through a no. 41 cellulose filter paper (Whatman) to obtain a golden-brown aqueous solution of pH 14. This metallofullerol solution was then passed down a Sephadex G 25–80 (Sigma) size-exclusion gel chromatography column using distilled H2O as the eluent. A single, colored band was eluted and collected. This solution (pH 6–7) was then filtered through a 0.22 μm Teflon (Acrodisc) filter, dried, weighed, and analyzed by ICP-AE and neutron-activation analysis for Ho content. This material was then stored as a dry solid in air. Exhaustive attempts to obtain mass spectrometry data for Hox@C82(OH)y by using the same techniques as for C60(OH)16 (53) were unsuccessful.
Preparation of 166Hox@C82(OH)y for Biodistribution Studies.
A 14.2-mg sample containing 165Hox@C82(OH)y was irradiated for 3 × 5 min irradiations. Between each of the irradiation periods, the sample was kept in a holding chamber cooled by flow of air for 15 min. This procedure was used to prevent long-term sample exposure to the high-temperature conditions at the irradiation position used (≈200°C). After the final irradiation, the sample was stored in a hot cell for 45 min before handling to allow decay of short-lived radioactive 28Al (t1/2 = 2.25 min) in the sample holder.
After irradiation, the sample was dissolved in 2 ml of distilled, deionized H2O. This dark brown solution was then passed through an AG 50 X4 cation exchange column (Na+ form, 100–200 mesh, ≈1-ml bed volume) to remove free Ho3+ liberated by sample decomposition during irradiation. Approximately 30% of the 166Ho passed through the column and thus was assumed to be intact metallofullerol. The column was then washed with an additional 1.6 ml of deionized H2O. The resulting effluent was filtered through a 0.22 μm Teflon Acrodisc filter to sterilize the sample and remove colloids. Finally, the solution was diluted to 4.0 ml with Mes buffer [2-(N-morpholino)-ethanesulfonic acid, Sigma) to maintain a biological pH. The total 166Ho activity of the metallofullerol solution was 200 μCi (1 Ci = 37 GBq).
Preparation of Na2[166Ho(DTPA)(H2O)] for Biodistribution Studies.
A sample containing 2.2 mg of radioactive 166Ho2O3 was dissolved in a mixture of 1 ml of concentrated HCl and 0.5 ml of HNO3. Thereafter, the solution was heated until clear and then evaporated to dryness. The residue was dissolved in 400 μl of 0.1 M HNO3. From this stock solution, 65 μl (1.89 × 10−3 mmol) of the solution was stirred with 190 μl of a 0.01 M sodium diethylenetriaminepentaacetate [Na5(DTPA)] solution (1.9 × 10−3 mmol/ml, Fluka). The solution was adjusted to pH 6 by the addition of 10 μl of 3 M NaOAc solution and was stirred for 10 minutes. The solution was then passed through a Chelex 100 column (≈0.5-ml bed volume, pre-equilibrated with NaOAc, pH 6.0) to remove any unchelated Ho3+. The sample next was diluted to 4.0 ml by addition of 3.75 ml of Mes buffer. The sample was then assayed in a well counter calibrated for the 166Ho radioisotope and found to contain 200 μCi of 166Ho activity. This preparation scheme is a slight modification of the published method (54) for producing Na2[Ln(DTPA)(H2O)] and as such, this solution can be regarded as equivalent to dissolving Na2[166Ho(DTPA)(H2O)] in buffer.
Biodistribution Studies Using BALB/c Mice.
Biodistribution studies of Na2[166Ho(DTPA)(H2O)] and 166Hox@C82(OH)y were performed on female, 7-week-old BALB/c mice. Twelve mice were used for the study of each material. The number of mice used was determined by the limited supply of Hox@C82(OH)y and the desire to also obtain metabolism data on Fisher rats (see below). Studies were conducted under approved protocols of the Institutional Animal Care and Use Committee at the Oak Ridge National Laboratory.
Mice were injected via a tail vein with 200 μl of the 166Ho solutions. Each injection contained approximately 10 μCi of 166Ho activity. At intervals of 1, 4, 24, and 48 h after injection, three mice were sacrificed, and samples of their organs were harvested in the following order: muscle (thigh), bone (whole leg with marrow), skin, uterus/ovaries, large intestine, stomach (emptied), liver, kidneys, spleen, fat (abdominal), thymus, heart, lungs, brain, and blood. Each organ was weighed, and the samples were analyzed for 166Ho activity. The fraction of injected radioactivity was calculated relative to external standard samples, which were evaluated at the beginning and end of each experimental set of samples.
Livers of the 166Hox@C82(OH)y-injected animals at the 48-h time point were harvested, homogenized separately, suspended in 15 ml of Mes buffer, and centrifuged at 5,000 rpm for 15 minutes. After centrifugation, the extraction was repeated. The 166Ho abundance in the pelleted material and in the extracted supernatant were then determined.
Metabolism Studies of 166Hox@C82(OH)y in Fischer Rats.
The remaining 166Hox@C82(OH)y stock solution from the biodistribution studies was passed through an AG50 4X cation exchange column and diluted to 1.8 ml with aqueous Mes buffer. A 500 μl (10 μCi) volume of the solution was then injected into two 12-week-old female Fischer rats through a tail vein. After administration of the metallofullerol, each rat was placed in a separate metabolism cage, where the animals’ feces and urine were collected. Feces and urine samples from each rat were analyzed for 166Ho content as described above for the mouse organs.
RESULTS AND DISCUSSION
Biodistribution Studies of 166Hox@C82(OH)y in BALB/c Mice.
The biodistribution of 166Hox@C82(OH)y was compared with that for Na2[166Ho(DTPA)(H2O)]. A Na2[166Ho(DTPA) (H2O)] control was chosen because of its similarity to the MRI contrast agent, Na2[Gd(DTPA)(H2O)], which clears from the body almost quantitatively in <1 h (<1% accumulation) (56).
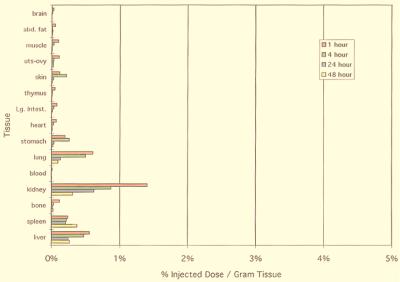
In the control study, a large fraction of Na2[166Ho(DTPA) (H2O)] was excreted from the mice within one hour of injection (Fig. 3). At this time point, the largest 166Ho concentration detected was in the kidney at 1.3% ID/g. After four hours, the total resident 166Ho in the organs assayed was <1% ID/g. This data agrees well with literature biodistribution data for Na2[Gd(DTPA)(H2O)] (54).
Figure 3.
Biodistribution of Na2[166Ho(DTPA)(H2O)] injected intravenously in 200 μl of Mes buffer into BALB/c Mice. The animals were sacrificed, and organs were sampled at 1, 4, 24, and 48 h, followed by measurement of the 166Ho 80.5 KeV γ-emission. Three animals were assayed at each time point, and reported values are the average of the % ID per g of tissue (σ range: 0.14–0.60% ID per g).
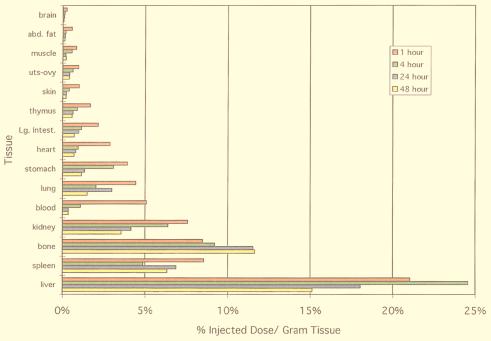
Biodistribution data for 166Hox@C82(OH)y in mice are summarized in Fig. 4. The data demonstrate that for times >1 h after injection, 166Ho was present in measurable levels throughout the entire body except in those tissues with limited blood flow such as the brain and fat. This long-term residency in the body (>1 h) is much longer than that observed for most metal chelates.
Figure 4.
Biodistribution of 166Hox@C82(OH)y in BALB/c mice at 1, 4, 24, and 48 h after injection. Conditions and details are the same as for Fig. 3 [σ range: 0.32–5.69% ID per g (liver and spleen) and 0.03–1.23% ID per g (other tissue)].
After 4 h, the concentration of 166Ho had dropped significantly in all organs except kidney, spleen, bone, and liver. Of these organs, only the liver and bone concentrations increased from 1 to 4 h. After 24 h, the concentration of the metallofullerol was lower in the liver and kidneys compared with the 4-h values. In contrast, there was a slow increase observed in bone concentration, with no evidence of clearance. At 48 h, the 166Ho concentration in the liver had decreased to 15% injected dose (ID) per g. This concentration was significantly lower than the maximum liver concentration at 24% ID per g. In addition, 166Ho concentrations had dropped to 0.36% and 3.6% ID per g in the blood pool and kidney, respectively. Therefore, the 166Ho tracer displays slow clearance from all tissue, except for the bone, which displayed a slight increase in concentration over 48 h.
One explanation for the accumulation of 166Ho in the liver and kidneys is that metallofullerols (or their aggregates) (55) are recognized by reticuloendothelial cells, and thus retained in the liver and spleen. Such behavior has been documented for lanthanide colloids, which give biodistribution data somewhat similar to that observed in this work (56, 57). The present study, however, indicates that metallofullerols are quite soluble (size <0.22 μm) at the concentrations administered, and that localization of 166Ho in the spleen is significantly lower than that found for traditional technetium and gold microcolloids (58, 59). Thus, there is a significant difference between the behavior of metallofullerols and the microcolloids. In any event, if metallofullerols eventually prove to be microcolloidal suspensions, they may improve on the properties of the colloids being examined as bone-targeting chemotherapy agents to treat leukemia, bone cancer and bone pain (56, 57). In fact, metallofullerols may have a special utility for this purpose, because empty fullerols (60) and other polyhydroxylated compounds (61, 62) have also demonstrated a high affinity for cortical bone.
The accumulation of free 166Ho3+ in the liver and bone would be predicted to resemble that of free metal ions (63) if the fullerene cage were metabolized and its internal Ho3+ were liberated. This possibility seems unlikely, however, in light of recent evidence that one C60 derivative is not metabolized in the liver after 5 days (39). Furthermore, data from the present work show that some of the 166Ho radiotracer recovered from the liver (see below) is most likely within the fullerol cage, and that the material secreted over 48 h also remains as the intact metallofullerene material. The demonstrated clearance in this work also differs from the behavior of free lanthanide (+3) metal ions, with one study showing no clearance from the liver (or bone) after 120 h (63).
To further explore the nature of the 166Ho accumulation in the liver, three mice were sacrificed 48 h after injection, and their livers were harvested, homogenized, and extracted with Mes buffer. The extract was then passed through a cation exchange column to determine whether 166Hox@C82(OH)y was still intact in solution. Data from this study indicates that only 25% of the 166Ho in the liver could be removed by buffered aqueous extraction. Almost all (95%) of this extracted 166Ho solution passed through a cation exchange column, indicating that the Ho3+ cation was nonionic, probably because it remained within the fullerol cage. The unextractable 166Hox@C82(OH)y in the liver could have undergone some metabolic oxidation, because fullerene oxidation has been observed for C60 derivatives in vivo (39), followed by selective absorption by liver cells (27, 29). It is also possible that residual metallofullerol remained physically trapped in the liver cell structure or within phagocytic cells. Future studies using longer lived metal isotopes (e.g., 170Tm) will focus on determining the ultimate fate of the entire dose of injected metallofullerol radiotracer. Such studies will also continue to probe the possibility of metabolism of the metallofullerol cage, because the question has not been resolved unambiguously by this first study.
Holmium metallofullerols are much more evenly distributed in the body than a radiolabeled trimethylene methane derivative of (empty) C60, which was retained mostly in the liver (90%), with little clearance after 48 h (26). In another biodistribution study of underivatized C60 in Swiss mice (4–5 mg/kg), it also was revealed that >95% of the compound was retained, mostly in the liver (22). Compared with these two studies, the metallofullerol studied here, 166Hox@C82(OH)y, displays much wider biodistribution and greater clearance. This observation offers encouragement for the future development of fullerene-based biological materials.
In the only other biodistribution study involving a metallofullerene, an insoluble suspension of 140La@C82 was injected directly into the heart of anaesthetized rats (25). After 24 h, >80% of the radioactivity still present in the subjects was located in the liver and blood pool, with some retention also in the brain. In contrast, Hox@C82(OH)y shows negligible accumulation in the brain after 24 h (<1% ID per g).
Metabolism Studies of 166Hox@C82(OH)y in Fischer Rats.
Immediately before the metabolism study, the metallofullerol sample was passed through a cation exchange column to determine whether any free Ho3+ had been liberated from the fullerol cage during the 10-h period between solution preparation and injection. Results from this study indicated that <1% of the 166Ho3+ had been released from the fullerol cage.
After injection of the two Fischer rats with 166Hox@C82 (OH)y, the feces and urine from the animals were collected every 24 h for 5 days. Radioisotope recovery data are summarized in Table 1 and clearly show that 166Hox@C82(OH)y exhibits its fastest clearance through the urine within 24 h. This result is consistent with the biodistribution data given herein, which shows that significant amounts of 166Hox@C82(OH)y had localized in the kidney but that the values for kidney-accumulated 166Ho dropped off quickly within the first 24 h. After the first day, however, Hox@C82(OH)y exhibited very slow clearance from the rat (≈1.5% per day), with nearly equal amounts in the feces and urine.
Table 1.
Excretion data of 166Ho from Fischer rats injected with 166Hox @ C82 (OH)y
| Excretion Day | 166Ho Feces excretion, % | 166Ho Urine excretion, % | 166Ho Cumulative dose remaining, % |
|---|---|---|---|
| 1 | 1.56 | 10.06 | 88.38 |
| 2 | 1.13 | 1.02 | 86.23 |
| 3 | 1.19 | 1.91 | 83.13 |
| 4 | 0.76 | 0.61 | 81.76 |
| 5 | 0.63 | 0.62 | 80.51 |
Values given are average %.
A complete pharmacokinetic study of C60-MSAD [MSAD, p,p′-bis(2-aminoethyl)-diphenyl-bis(monosuccinimide)] has been performed by Schinazi and coworkers (30). In their work, the empty fullerene derivative displayed a clearance half-life of 6.8 ± 1.1 h [a much faster rate than for 166Hox@C82(OH)y]. The compound also displayed a low toxicity but no renal excretion after 24 h. The same study also demonstrated nearly 100% of the C60-MSAD bound to protein in vitro. This binding interaction is thought to be the primary reason that C60-MSAD [and now possibly Hox@C82(OH)y] is excreted slowly. Additionally, C60-MSAD seemed to be tolerated in rats up to 25 mg/kg, but above this concentration, the subjects died within 5 min. This concentration is at least an order of magnitude higher than the dosage of Hox@C82(OH)y used in this study, so no acute toxicity comparison can be made.
CONCLUSIONS
Biodistribution studies of 166Hox@C82(OH)y in BALB/c mice over a 48-h period have demonstrated the feasibility of using endohedral metallofullerene compounds as radiotracers for in vivo studies of fullerene-based materials. In particular, the present studies have established that metallofullerols (i) have a blood pool residency time of over an hour with nearly total clearance from blood shortly thereafter, (ii) localize in liver but with continued slow excretion, (iii) are likely unmetabolized (at least 25%) in the liver, (iv) localize and are retained in bone, and (v) were not acutely toxic in vivo at the doses tested. Slow but steady clearance of the metallofullerol over 5 days was also noted in a metabolism study using Fischer rats. These results indicate that the clearance pathways, residency times, and tissue distribution for metallofullerols make them and other fullerol-like materials potential candidates for biomedical applications, as already suggested by Dugan and coworkers for C60(OH)y and C60[C(COOH)2]3 (17, 38). Future efforts will undoubtedly produce more sophisticated fullerene-based materials for various biological applications, and metallofullerene radiotracers will likely continue to play a central role in elucidating their behavior in animals.
Acknowledgments
D.W.C. thanks Thomas P. Thrash (also at Rice) for his collaboration and many fruitful discussions, which helped lay the groundwork for this study. We also thank Dr. Gary Ehrhardt of Missouri University Research Reactor at the University of Missouri for insightful contributions to many aspects of the project. At TDA Research (J.M.A.) and Rice University (L.J.W.), funding for this project was provided by a National Institutes of Health–Small Business Innovation Research Grant (IR43CA73245). At Rice University, L.J.W. thanks the Robert A. Welch Foundation (Grant C-0627) for partial support. Research conducted at Oak Ridge National Laboratory was supported by the Office of Biological and Environmental Research, U.S. Department of Energy, under contract DC-AC05-960R22464 with Lockheed Martin Energy Research Corporation (S.M. and S.J.K.).
ABBREVIATIONS
- LD-TOF
laser desorption–time-of-flight
- ICP
inductively coupled plasma
- AE
atomic emission
- NAA
neutron-activation analysis
- DTPA
diethylenetriaminepentaacetate
- ID
injected dose
Footnotes
Based on the LD-TOF MS of HPLC-purified holmium fullerenes used in this study (Fig. 2), it can be seen that the sample contained both mono- and diholmium metallofullerene. Therefore, the molecular formula for these compounds has been noted with an “x” subscript where x = 1 or 2.
As a result of recent work concerning the number hydroxyl groups present for C60(OH)y, the number of hydroxyl groups on holmium metallofullerol is currently assigned as 16 (see ref. 52), although it may well be different for a metallofullerene with a C82 cage. Exhaustive attempts to obtain mass spectrometry data for Hox@C82(OH)y by using the same techniques as in ref. 52 were unsuccessful.
References
- 1.Kroto H W, Heath J R, O’Brien S C, Curl R F, Smalley R E. Nature (London) 1985;318:162–163. [Google Scholar]
- 2.Heath J R, O’Brien S C, Zhang Q, Liu L, Curl R F, Kroto H W, Tittel F K, Smalley R E. J Am Chem Soc. 1985;107:7779–7780. [Google Scholar]
- 3.Chen B-X, Wilson S R, Das M, Coughlin D J, Erlanger B F. Proc Natl Acad Sci USA. 1998;95:10809–10813. doi: 10.1073/pnas.95.18.10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirsch A. Angew Chem Int Ed Engl. 1993;32:1138–1141. [Google Scholar]
- 5.Bethune D S, Johnson R D, Salem J R, de Vries M S, Yannoni C S. Nature (London) 1993;366:123–128. [Google Scholar]
- 6.Shinohara H, Sato H, Saito Y, Ohkohchi M, Ando Y. J Phys Chem. 1992;96:3571–3573. [Google Scholar]
- 7.Bandow S, Kitagawa H, Mitani T, Inokuchi H, Saito Y, Yamaguchi H, Hayashi N, Sato H, Shinohara H. J Phys Chem. 1992;96:9609–9612. [Google Scholar]
- 8.Shinohara H, Yamaguchi H, Hayashi N, Sato H, Inagaki M, Saito Y, Bandow S, Kitagawa H, Mitani T, Inokuchi H. Mater Sci Eng B. 1993;19:25–30. [Google Scholar]
- 9.Yamamoto K, Funasaka H, Takahashi T, Akasaka T. J Phys Chem. 1994;98:2008–2011. [Google Scholar]
- 10.Kikuchi K, Suzuki S, Nakao Y, Nakahara N, Wakabayashi T, Shiromaru H, Saito K, Ikemoto I, Achiba Y. Chem Phys Lett. 1993;216:67–71. [Google Scholar]
- 11.Akasaka T, Kato T, Kobayashi K, Nagase S, Yamamoto K, Funasaka H, Takahashi T. Nature (London) 1995;374:600–601. [Google Scholar]
- 12.Suzuki T, Maruyama Y, Kato T, Akasaka T, Kobayashi K, Nagase S, Yamamoto K, Funasaka H, Takahashi T. J Am Chem Soc. 1995;117:9606–9607. [Google Scholar]
- 13.Akasaka T, Nagase S, Kobayashi K, Suzuki T, Kato T, Kikuchi K, Achiba Y, Yamamoto K, Funasaka H, Takahashi T. Angew Chem Int Ed Engl. 1995;34:2139–2141. [Google Scholar]
- 14.Akasaka T, Kato T, Nagase S, Kobayashi K, Yamamoto K, Funasaka H, Takahashi T. Tetrahedron. 1996;52:5015–5020. [Google Scholar]
- 15.Zhang S, Sun D, Li X, Pei F, Liu S. Fullerene Sci Technol. 1997;5:1635–1643. [Google Scholar]
- 16.Unger E D, Shen K, Wu G, Fritz T. Magn Res Med. 1991;22:304–308. doi: 10.1002/mrm.1910220229. [DOI] [PubMed] [Google Scholar]
- 17.Dugan L L, Turetsky D M, Du C, Lobner D, Wheeler M, Almli C R, Shen C K-F, Luh T-Y, Choi D W, Lin T-S. Proc Natl Acad Sci USA. 1997;94:9434–9439. doi: 10.1073/pnas.94.17.9434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun D, Liu Z, Guo X, Liu S. Chin J Appl Chem. 1996;13:1–4. [Google Scholar]
- 19.Tsuchiya T, Yamakoshi Y N, Miyata N. Biochem Biophys Res Comm. 1995;206:885–894. doi: 10.1006/bbrc.1995.1126. [DOI] [PubMed] [Google Scholar]
- 20.Pukhova Ya I, Churliov G N, Isakova V G, Korets A Ya, Titarenko, Ya N. Dokl Biochem (Transl of Dokl Akad Nauk) 1997;355:269–272. [PubMed] [Google Scholar]
- 21.Baieri T, Drosselmeyer E, Seidel A, Hippeli S. Exp Toxicol Pathol. 1996;48:508–511. doi: 10.1016/S0940-2993(96)80068-6. [DOI] [PubMed] [Google Scholar]
- 22.Satoh M, Matsuo K, Kiriya H, Mashino T, Nagano T, Hirobe M, Takayanagi I. Eur J Pharmacol. 1997;327:175–181. doi: 10.1016/s0014-2999(97)89658-6. [DOI] [PubMed] [Google Scholar]
- 23.Moussa F, Trivin F, Céolin R, Hadchouel M, Sizaret P Y, Greugny V, Fabre C, Rassat A, Szwarc H. Fullerene Sci Technol. 1996;4:21–29. [Google Scholar]
- 24.Tokuyama H, Yamago S, Nakamura E, Shiraki T, Sugiura Y. J Am Chem Soc. 1993;115:7918–7919. [Google Scholar]
- 25.Kobayashi K, Kuwano M, Sueki K, Kikuchi K, Achiba Y, Nakahara H, Kananishi N, Wantanabe M, Tomura K. J Radioanal Nucl Chem. 1995;192:81–89. [Google Scholar]
- 26.Yamago S, Tokuyama H, Nakamura E, Kikuchi K, Kananishi S, Sueki K, Nakahara H, Enomoto S, Ambe F. Chem Biol. 1995;2:385–389. doi: 10.1016/1074-5521(95)90219-8. [DOI] [PubMed] [Google Scholar]
- 27.Schinazi F F, Bellavia C, Gonzalez R, Hill C L, Wudl F. In: Fullerenes: Recent Advances in the Chemistry and Physics of Fullerenes and Related Materials. Kadish K M, Ruoff R S, editors. Vol. 2. Pennington, NJ: Electrochem. Soc.; 1995. pp. 696–698. [Google Scholar]
- 28.Schinazi R F, McMillan A, Juodawlkis A S, Pharr J, Sijbesma R, Srdanov G, Hummelen J-C, Boudinot F D, Hill C L, Wudl F. In: Fullerenes: Recent Advances in the Chemistry and Physics of Fullerenes and Related Materials. Kadish K M, Ruoff R S, editors. Vol. 1. Pennington, NJ: Electrochem. Soc.; 1994. pp. 689–696. [Google Scholar]
- 29.Schinazi R F, Sijbesma R, Srdanov G, Hill C L, Wudl F. Antimicrob Agents Chemother. 1993;37:1707–1710. doi: 10.1128/aac.37.8.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajagopalan P, Wudl F, Schinazi R F, Boudinot F D. Antimicrob Agents Chemother. 1996;40:2262–2265. doi: 10.1128/aac.40.10.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sijbesma R, Srdanov G, Wudl F, Castoro J A, Wilkins C, Friedman S H, DeCamp D, Kenyon G L. J Am Chem Soc. 1993;115:6510–6512. [Google Scholar]
- 32.Schinazi R, Chiang L, Wilson L J, Cagle D W, Hill C L. In: Fullerenes: Recent Advances in the Chemistry and Physics of Fullerenes and Related Materials. Kadish K M, Ruoff R S, editors. Vol. 4. Pennington, NJ: Electrochem. Soc.; 1997. pp. 357–360. [Google Scholar]
- 33.Friedman S H, DeCamp D L, Sijbesma R P, Srdanov G, Wudl F, Kenyon G L. J Am Chem Soc. 1993;115:6506–6509. [Google Scholar]
- 34.Toniolo C, Bianco A, Maggini M, Scorrano G, Prato M, Marastoni M, Tomatis R, Spisani S, Palú G, Blair E. J Med Chem. 1994;37:4558–4562. doi: 10.1021/jm00052a015. [DOI] [PubMed] [Google Scholar]
- 35.Chiang L Y, Lu F-J, Lin J-T. In: Fullerenes: Recent Advances in the Chemistry and Physics of Fullerenes and Related Materials. Kadish K M, Ruoff R S, editors. Vol. 2. Pennington, NJ: Electrochem. Soc.; 1995. pp. 699–706. [Google Scholar]
- 36.Miyata N, Yamakoshi Y. In: Fullerenes: Recent Advances in the Chemistry and Physics of Fullerenes and Related Materials. Kadish K M, Ruoff R S, editors. Vol. 5. Pennington, NJ: Electrochem. Soc.; 1997. pp. 345–357. [Google Scholar]
- 37.Okuda K, Hirobe M, Mochizuki M, Mashino T. In: Fullerenes: Recent Advances in the Chemistry and Physics of Fullerenes and Related Materials. Kadish K M, Ruoff R S, editors. Vol. 5. Pennington, NJ: Electrochem. Soc.; 1997. pp. 337–344. [Google Scholar]
- 38.Dugan L L, Gabrielson J K, Yu S, Lin T-S, Choi D W. Neurobiol Dis. 1996;3:129–135. doi: 10.1006/nbdi.1996.0013. [DOI] [PubMed] [Google Scholar]
- 39.Bullard-Dillard R, Creek K E, Scrivens W A, Tour J M. Bioorg Chem. 1996;24:376–385. [Google Scholar]
- 40.Lasic D D, Needham D. Chem Rev. 1995;95:2601–2628. [Google Scholar]
- 41.Du H, Chandaroy P, Hui S W. Biochim Biophys Acta. 1997;1326:236–248. doi: 10.1016/s0005-2736(97)00027-8. [DOI] [PubMed] [Google Scholar]
- 42.Mumper R J, Ryo U Y, Jay M. J Nucl Med. 1991;32:2139–2143. [PubMed] [Google Scholar]
- 43.Dadachova E, Mirzadeh S, Lambrecht R M, Hetherington E L, Knapp F F., Jr Anal Chem. 1994;66:4272–4277. [Google Scholar]
- 44.Dadachova E, Mirzadeh S, Smith S V, Knapp F F, Jr, Hetherington E L. Appl Radiat Isot. 1997;48:477–481. doi: 10.1016/s0969-8043(96)00269-2. [DOI] [PubMed] [Google Scholar]
- 45.Gillan E G, Yeretzian C, Min K S, Alvarez M M, Whetten R L, Kaner R B. J Phys Chem. 1992;96:6869–6871. [Google Scholar]
- 46.Bartl A, Dunsch L, Kirbach U. Proc SPIE-Int Soc Opt Eng. 1996;2854:17–27. [Google Scholar]
- 47.Wan T S M, Zhang H-W, Tso T S C, Kwong K P, Wong T. In: Fullerenes: Recent Advances in the Chemistry and Physics of Fullerenes and Related Materials. Kadish K M, Ruoff R S, editors. Vol. 4. Pennington, NJ: Electrochem. Soc.; 1997. pp. 490–506. [Google Scholar]
- 48.Wang W, Ding J, Yang S, Li X-Y. In: Fullerenes: Recent Advances in the Chemistry and Physics of Fullerenes and Related Materials. Kadish K M, Ruoff R S, editors. Vol. 4. Pennington, NJ: Electrochem. Soc.; 1997. pp. 417–428. [Google Scholar]
- 49.Lawrence K L S, Ehrhardt G J, Cagle D W, Thrash T P, Wilson L J. In: Fullerenes: Recent Advances in the Chemistry and Physics of Fullerenes and Related Materials. Kadish K M, Ruoff R S, editors. Vol. 2. Pennington, NJ: Electrochem. Soc.; 1995. pp. 66–71. [Google Scholar]
- 50. Thrash T P, Cagle D W, Alford M, Erhardt G J, Lattimer J C, Wilson L J. In: Fullerenes: Recent Advances in the Chemistry and Physics of Fullerenes and Related Materials. Kadish K M, Ruoff R S, editors. Vol. 4. Pennington, NJ: Electrochem. Soc.; 1997. pp. 349–356. [Google Scholar]
- 51.Cagle D W, Thrash T P, Alford M, Chibante L P F, Ehrhardt G J, Wilson L J. J Am Chem Soc. 1996;118:8043–8047. [Google Scholar]
- 52.Chiang L Y, Bhonsle J B, Wang L, Shu S F, Chang T M, Hwu J R. Tetrahedron. 1996;52:4963–4972. [Google Scholar]
- 53.Li, J., Takeuchi, A., Ozawa, M., Li, X., Saigo, K. & Kitazawa, K., (1993) J. Chem. Soc. Chem. Commun., 1784–1785.
- 54.Prato F S, Wisenberg G, Marshall T P, Uksik P, Zabel P. J Nucl Med. 1988;29:1683–1687. [PubMed] [Google Scholar]
- 55.Mohan H, Palit D K, Mittal J P, Chiang L Y, Asmus K-D, Guldi D M. J Chem Soc Faraday Trans. 1998;94:359–363. [Google Scholar]
- 56.Parks N J, Kawakami T G, Avila M J, White R, Cain G R, Raaka S D, Hornoff W, Fisher P, Moore P, Seibert J A, et al. Blood. 1993;82:318–325. [PubMed] [Google Scholar]
- 57.Desrosiers M F, Avila M J, Schauer D A, Coursey B M, Parks N J. Appl Radiat Isot. 1993;44:459–463. doi: 10.1016/0969-8043(93)90267-e. [DOI] [PubMed] [Google Scholar]
- 58.Mundschenck H, Hromac A, Fischer J. J Nucl Med. 1971;12:711–718. [PubMed] [Google Scholar]
- 59.Carter T L, Ankeney J L. J Nucl Med. 1964;5:901–912. [PubMed] [Google Scholar]
- 60.Gonzalez K A. Ph.D. dissertation. Houston: Rice University; 1998. [Google Scholar]
- 61.Willson T M, Charifson P S, Baxter A D, Geddie N G. Bioorg Med Chem Lett. 1996;6:1043–1046. [Google Scholar]
- 62.Willson T M, Henke B R, Momtahen T M, Garrison D T, Moore L B, Geddie N G, Baer P G. Bioorg Med Chem Lett. 1996;6:1047–1050. [Google Scholar]
- 63.Smith S V, Di Bartollo N, Mirzadeh S, Lambrecht R M, Knapp F F R, Jr, Hetherington E L. Appl Radiat Isot. 1995;46:759–764. doi: 10.1016/0969-8043(94)00149-t. [DOI] [PubMed] [Google Scholar]