Abstract
Our development of vaccines to prevent shigellosis is based on the hypothesis that a critical (protective) level of serum IgG to the O-specific polysaccharide (O-SP) domain of Shigella lipopolysaccharide (LPS) confers immunity. The O-SP is a hapten and must be conjugated to a protein to induce serum antibodies. The O-SP of Shigella dysenteriae type 1 (≈27 tetrasaccharide repeat units), prepared by acid hydrolysis of the LPS, was bound to human serum albumin (HSA) by multiple point attachment (O-SP-HSA): The molar ratio of HSA to O-SP was 1.0. Synthetic saccharides, composed of one or multiples of the O-SP tetrasaccharide, equipped with a spacer at their reducing end, were bound to HSA by a single point attachment: The average molar ratios of the saccharides to HSA ranged from 4 to 24. Serum IgG anti-LPS, elicited in mice by O-SP-HSA or synthetic tetra-, octa-, dodeca-, and hexadecasaccharide fragments, was measured by ELISA. Outbred 6-week-old female mice were injected s.c. three times at biweekly intervals with 2.5 μg of saccharide as a conjugate and were bled 7 days after the second and third injections. Excepting the tetramer, conjugates of the octamer, dodecamer and hexadecamer elicited IgG LPS antibodies after the second injection, a statistically significant rise (booster) after the third injection, and higher levels than those vaccinated with O-SP-HSA (P = 0.0001). The highest geometric mean levels of IgG anti-LPS were elicited by the hexadecamer with 9 chains or 9 moles of saccharide/HSA (15.5 ELISA units) followed by the octamer with 20 chains (11.1 ELISA units) and the dodecamer with 10 chains (9.52 ELISA units). Clinical evaluation of these synthetic saccharides bound to a medically useful carrier is planned.
There are as yet no licensed vaccines for prevention of shigellosis, despite the discovery of shigellae as a common and serious human pathogen about one century ago (1). Within the genus of Shigella, Shigella dysenteriae type 1 (Shiga’s Bacillus) causes both endemic and epidemic shigellosis with serious extraintestinal complications, including the hemolytic uremic syndrome, accompanied by a high rate of mortality: Today the organism manifests almost universal antibiotic resistance (2–7). In addition, S. dysenteriae type 1 is unique among shigellae in that it alone expresses an exotoxin called Shiga toxin. Shiga toxins, also expressed by several Escherichia coli, including lipopolysaccharide (LPS) type O157, have been correlated with the development of hemolytic uremic syndrome (8, 9). Because of their similarities, shigellae and E. coli should be considered as belonging to one genus (10). If our approach to inducing immunity to S. dysenteriae type 1 by stimulating serum IgG anti-O-specific polysaccharide (anti-O-SP) is successful, it should be applicable to all shigellae and to E. coli (10–12).
Although the O-SP domain of the Shigella LPS has been shown to be both an essential virulence factor and a protective antigen, there is no consensus about the nature of host immunity, and there is no valid animal model or in vitro bioassay that has been correlated with protection against shigellosis (11). It has been hypothesized that a critical (protective) level of serum IgG anti-O-SP confers immunity to shigellosis by killing the inoculum of Shigella on the epithelial surface of the small intestine (11–13). O-SPs are nonimmunogenic because of their low molecular weights (hapten). Conversion to immunogens has been achieved by binding the O-SPs of S. dysenteriae type 1, Shigella flexneri type 2a, and Shigella sonnei to proteins (14, 15). These conjugates did not elicit fever or significant local reactions in adult volunteers. In one study, O-SP protein conjugates of S. sonnei and of S. flexneri type 2a elicited levels of IgG anti-LPS similar to those in patients convalescent from shigellosis (15). The S. dysenteriae type 1 O-SP conjugates induced a ≥4-fold rise of IgG anti-LPS in 59% of U.S. Army recruits (15). In a randomized, double-blinded, vaccine-controlled trial, S. sonnei O-SP conjugated to the nontoxic recombinant exoprotein A of Pseudomonas aeruginosa was effective (74%) in preventing shigellosis in Israeli Armed Forces recruits during their basic training in the summer: The efficacy was related to the level of IgG anti-LPS elicited by the S. sonnei O-SP–recombinant exoprotein A vaccine (16). This conjugate also prevented shigellosis’ occurring within 1 to 17 days after vaccination, albeit at a lower rate (43%), indicating that this type of vaccine could be useful in controlling epidemics. Our objective is to develop conjugates that elicit high and sustained levels of IgG anti-LPS in infants as part of their routine immunization, as has been achieved with conjugates of the Haemophilus influenzae type b capsular polysaccharide (17, 18).
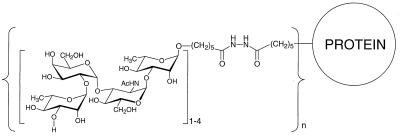
The O-SP of S. dysenteriae type 1 is composed of a tetrasaccharide repeating unit (19): 3)-α-l-Rhap-(1→3)-α-l-Rhap-(1→2)-α-d-Galp-(1→3)-α-d-GlcpNAc-(1→. Mapping of the carbohydrate-binding specificities of a murine mAb to this O-SP has been reported (20). Saccharides corresponding to the O-SP have been synthesized, including tetra-, octa-, dodeca-, and hexadecasaccharides with a spacer at their reducing end, and were bound to HSA by single point attachment technique developed in our laboratory (21–27). The schematic structure of these conjugates is shown in Fig. 1. We report the serum IgG anti-LPS responses elicited by s.c. injection of saline solutions of these conjugates, according to a clinically relevant dosage and vaccination scheme, into 6-week-old outbred female mice (14, 17).
Figure 1.
Schematic structure of the HSA conjugates of the synthetic saccharides. The tetrasaccharide repeat unit of the O-SP of S. dysenteriae type 1, 3)-α-l-Rhap-(1→2)-α-d-Galp-(1→3)-α-d-GlcpNAc-(1→3)-α-l-Rhap-(1→, is shown with the spacer at its reducing end. The tetrasaccharides for the octamer, dodecamer and hexadecamer were added to the nonreducing end in prefabricated form.
METHODS
Saccharides.
The synthesis and characterization of a tetra-, octa-, dodeca-, and hexadecasaccharide fragment of the O-SP, with a spacer at their reducing ends, have been reported (25–26). The LPS of S. dysenteriae type 1 was prepared, and its O-SP was purified as described (14). The resultant product contained <1% of protein or nucleic acid and had MW of 21.4 (≈27 repeat units) as estimated by gel filtration. Silver stain PAGE of 10 μg showed no LPS.
HSA.
HSA (Sigma) was treated by diafiltration against ion-exchanged water at room temperature and was sterile-filtered and freeze-dried.
Conjugates (Table 1).
Table 1.
Compositional analyses of the conjugates
| Conjugate code | Carbohydrate chain length | Carbohydrate chains/HSA | MW | HSA/carbohydrate, wt/wt | Carbohydrate, wt % |
|---|---|---|---|---|---|
| IV/4 | 4 | 11 | 78 | 7.8 | 13 |
| VIII/8 | 8 | 11 | 83 | 3.9 | 20 |
| VIII/20 | 8 | 20 | 98 | 2.1 | 30 |
| XII/8 | 12 | 8 | 84 | 4.2 | 18 |
| XII/10 | 12 | 10 | 88 | 3.1 | 24 |
| XII/23 | 12 | 23 | 118 | 1.3 | 43 |
| XVI/4 | 16 | 4 | 78 | 5.6 | 15 |
| XVI/9 | 16 | 9 | 93 | 2.5 | 28 |
| XVI/19 | 16 | 19 | 120 | 1.2 | 45 |
| O-SP | ≈27 | N.A. | N.A. | 1.0 | 51 |
The conjugates are identified by the length of the saccharide (Roman numerals) and the average number of saccharides per HSA (Arabic numerals). N.A., not applicable.
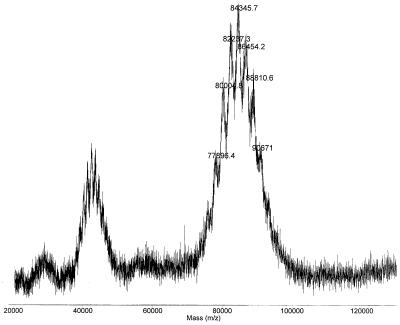
The composition of each of nine synthetic saccharide-HSA conjugates was calculated from the spectra obtained by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (26). A representative mass spectrum of the XVI/8 conjugate is shown in Fig. 2. The center peak in the singly charged cluster at m/z 84.3 kDa corresponds to a conjugate having an average of eight saccharide chains per molecule of HSA, the average number of saccharides per HSA. The molecular mass of HSA was 66.35 kDa measured by this technique.
Figure 2.
Matrix-assisted laser desorption ionization time-of-flight mass spectrum of a dodecasaccharide-HSA conjugate. The average MW is 84.3 with a range of 77–91, corresponding to an average of eight saccharide chains per HSA.
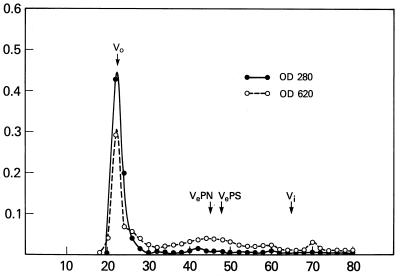
O-SP was activated with cyanogen bromide, was derivatized with adipic acid dihydrazide (3.0% wt/wt), and was bound to HSA by carbodiimide condensation (14). The reaction mixture contained equal amounts of HSA and adipic hydrazide derivative of the O-SP (each 10 mg/ml). The pH was brought to 5.3 by addition of 0.2 M HCl in a pH-stat. 1-Ethyl-3-dimethylaminopropylcarbodiimide was added, and the reaction mixture was maintained at pH 5.3 for 4 hours. The reaction mixture was dialyzed against 0.2 M NaCl overnight at 4°C and was applied to a 1.0- × 90-cm column of CL-6B Sepharose in 0.2 M NaCl. Fractions that emerged in the void volume of the column were pooled and used for vaccination of mice (Fig. 3). In separate experiments with this column, the O-SP eluted with a partition coefficient (Kd) of 0.47, and the HSA eluted with a Kd of 0.57. The composition of the O-SP-HSA conjugate, determined by chemical analyses, was 1.0 O-SP/protein (wt/wt) (14).
Figure 3.
The reaction mixture of O-SP adipic hydrazide, HSA, and 0.1 M 1-ethyl-3-dimethylaminopropylcarbodiimide at room temperature for 4 hours with pH maintained at 5.25 was dialyzed against 0.2 M NaCl for 2 days at 4–7°C and was applied to a 1- × 95-cm column of CL-6B Sepharose column in 0.2 M NaCl, and 3.5-ml fractions were collected. Saccharide was determined by the anthrone reaction (14), and protein was determined by A280. Void volume fractions were taken as conjugate: O-SP–adipic hydrazide and HSA had Kds of 0.4 and 0.5, respectively.
Immunization.
Groups of 20 6-week-old female Swiss–Webster mice were injected s.c. with 2.5 μg of saccharide as a conjugate every 2 weeks: groups of 10 were exsanguinated 7 days after the second and the third injections (14, 17). Four independent experiments were done. Controls were mice injected with saline.
ELISA.
Immulon 4-microtiter plates were coated with 100 μl of a solution containing 10 μg/ml of S. dysenteriae type 1 LPS, and ELISA was performed as described (15). The results were calculated as a percent of a high-titered reference serum, arbitrarily assigned a value of 100 ELISA units (EU) by parallel line analysis with a program of the Centers for Disease Control, and expressed as the geometric mean (28).
Statistical Analysis.
Comparisons of geometric means were performed by unpaired t tests. The Statistical Analysis System (SAS Institute, Cary, NC) was used for all data analysis.
RESULTS AND DISCUSSION
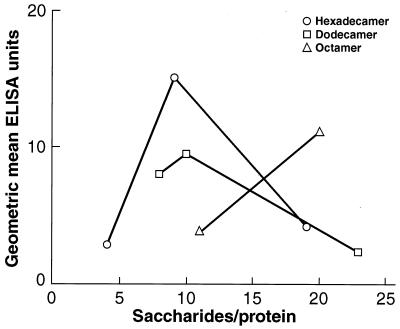
Serum IgG Anti-LPS (Table 2 and Fig. 4).
Table 2.
Geometric mean serum IgG anti-LPS levels (ELISA units) after the third injection of S. dysenteriae type 1 conjugates into mice*
| Number of experiments | Immunogen | Carbohydrates/protein, mol/mol | IgG anti-LPS |
|---|---|---|---|
| 3 | Octamer–HSA | 11 | 3.77 |
| 2 | Octamer–HSA | 20 | 11.1 |
| 4 | Dodecamer–HSA | 8 | 8.14 |
| 2 | Dodecamer–HSA | 10 | 9.52 |
| 2 | Dodecamer–HSA | 23 | 2.18 |
| 2 | Hexadecamer–HSA | 4 | 2.60 |
| 4 | Hexadecamer–HSA | 9 | 15.5 |
| 2 | Hexadecamer–HSA | 19 | 4.13 |
| 2 | O-SP-HSA | N.A. | 0.91 |
In 4 separate experiments, groups of 20 female 6-week-old general purpose mice from the NIH colony were injected s.c. with 2.5 μg of saccharide three times 2 weeks apart, and 10 from each group were exsanguinated 7 days after the second or third injections. Only the geometric mean IgG anti-LPS levels after the third injection of the conjugates are shown. Mice injected with saline or O-SP alone had no detectable antibody (data not shown). 11.1 vs. 3.77; P = 0.0003, 9.52, 8.14 vs. 2.18; P < 0.001; 15.5 vs. 2.60, 4.13; P = 0.0001; 15.5 vs. 8.14; P = 0.02, 15.5 vs. 9.52; NS. N.A., not applicable.
Excluding the tetramer.
Figure 4.
Geometric mean of serum IgG anti-LPS (EU) 7 days after the third s.c. injection, 2 weeks apart, of 2.5 μg of saccharide as conjugates into 6-week-old female outbred mice. Conjugates of varying saccharide chain length and density (tetramer excluded) were injected in four experiments.
Neither saline nor the O-SP alone elicited IgG anti-LPS (data not shown). All of the synthetic conjugates elicited low levels of anti-LPS after the second injection that were similar among the groups. Accordingly, only the levels of IgG anti-LPS after the third injection are shown.
With the exception of the tetramer, all of the synthetic saccharide conjugates elicited statistically significant rises of IgG anti-LPS after the third injection, and all elicited higher levels of IgG anti-LPS than the conjugates prepared with the O-SP (P = 0.0001). In the four experiments, each conjugate elicited IgG anti-LPS levels that, for each conjugate, were not statistically significantly different from each other.
The tetramer with 11 chains (moles of saccharide/mole of HSA) elicited low levels of anti-LPS (0.60 EU) in the first experiment and was not evaluated further. The octamer with 11 chains elicited 3.77 EU in three experiments, and that with 20 chains elicited 11.1 EU in two experiments: The difference between these two levels was significant (11.1 vs. 3.77; P = 0.0003).
With the dodecamer, the highest level of IgG anti-LPS was elicited by the conjugate with 10 chains, but this level was not significantly different from that elicited by the one with 8 chains (9.52 vs. 8.14; P not significant). These two conjugates elicited higher levels of IgG anti-LPS than the dodecamer with 23 chains (8.14, 9.52 vs. 2.18; P < 0.001).
Of three hexadecamer conjugates, that with nine chains elicited the highest level of IgG anti-LPS (15.5 vs. 2.60, 4.13; P = 0.0001): There was no significant difference between the levels elicited by the conjugate with 4 chains or with 19 chains.
The hexadecamer with 9 chains elicited the highest level of IgG anti-LPS (15.5 EU), the second was the octamer, with 20 chains (11.1 EU), and the third was the dodecamer, with 10 chains (9.52 EU) (15.5 vs. 11.1, 9.52; P not significant).
Serum IgG antibodies to the homotypic capsular polysaccharide confers immunity to H. influenzae type b, meningococci, pneumococci, Salmonella typhi, or group B streptococci (13,17,18). Similarly, seroepidemiologic studies and a clinical trial with a S. sonnei O-SP conjugate provided evidence that the level of serum IgG anti-LPS is related to immunity to shigellosis (11, 13–16, 29, 30). Improved immunogenicity of our Shigella conjugates is sought so that they may be more effective and can be administered as part of the routine immunization of infants. In this report, HSA conjugates of synthetic saccharides elicited higher levels of serum IgG anti-LPS in young outbred mice than did conjugates prepared with the O-SPs purified from the LPS of S. dysenteriae type 1 (14, 15). Synthetic oligosaccharides, corresponding to the structure of H. influenzae type b polysaccharide, and their conjugation to proteins or T-cell-dependent peptides have been reported (31). The resultant conjugates elicited serum antibodies in mice and in monkeys comparable to those after injection of the licensed conjugate vaccine. We suggest that the synthetic approach may be applicable to all polysaccharide-based vaccines.
The immunogenicity of saccharides, alone or as protein conjugates, is related to several variables: (i) the age of the recipient (17); (ii) the immunologic properties of the saccharide and of the protein (32); (iii) the minimal size of the saccharide required to induce antibodies; (33–36); (iv) a minimal density of the saccharide on the protein (36, 37); and (v) configuration of the conjugate (single vs. multiple point attachment) (17). Attempts to optimize the immunogenicity of saccharides must take these variables into account and should evaluate their potential by administration to humans and to animals with dosages, formulations, and vaccination routes that are clinically relevant.
Both the molecular weight and density of the saccharides on the protein were related to the immunogenicity of the conjugate. The hexadecamer was the most immunogenic; the small increment in immunogenicity of the hexadecamer over the dodecamer at comparable densities suggests that there will be little to gain by increasing further the length of these saccharides. Optimal immunogenicity was achieved for the hexadecamer and dodecamer at about nine chains per molecule of protein. Lower levels of IgG anti-LPS were elicited at both lower and higher densities. The use of synthetic saccharides permitted the identification of two variables, chain length and chain density, that determined the immunogenicity of the conjugates. Saccharides, prepared from hydrolysis of polysaccharides subjected to gel filtration, are heterogeneous with respect to their molecular weight (MW) and end groups; that is, low MW components of “high” MW fractions overlap with high MW components of “low” MW fractions. Conjugation to a protein could favor one species, such as lower-MW saccharides, that could explain the difficulty in distinguishing these two variables as determinants of immunogenicity (38, 39).
The immunogenicity of our synthetic saccharide conjugates likely involves the interaction of both B-cells and T-cells (40). Our interpretation of the IgG anti-LPS response is as follows: (i) The hexadecamer has a higher affinity for the antibody-combining site of B-cell membrane IgM receptors, resulting in more effective clustering and signal transduction (35–38); (ii) a lower density of saccharides per protein (eight for the dodecamer and four for the hexadecamer) resulted in a less-than-optimal antigenic multivalency for receptor clustering; and (iii) a higher density of saccharides (23 for the dodecamer and 19 for the hexadecamer) “covers” the protein, resulting in a lower number of peptide B-cell MHC ligands available for stimulating T-cells. This “covering” effect is also related to the length of the saccharide because the octamer with 20 chains per HSA was also an effective immunogen.
Conjugates, prepared by binding capsular polysaccharides and O-SPs at multiple sites to proteins, have been evaluated clinically (17). The composition of these conjugates is varied, and there is yet no physicochemical method to predict reliably their immunogenicity (standardization). This problem may be overcome by using synthetic saccharides of defined size and composition and with a single active site for conjugation, as has been achieved for the S. dysenteriae type 1 O-SP. The structure of the resultant conjugate can be characterized more accurately by matrix-assisted laser desorption ionization time-of-flight mass spectrometry than can be achieved by chemical analysis and gel filtration.
Tetrasaccharide units are assembled for blockwise construction of the octamer, dodecamer, and hexadecamer (21–27). With the single attachment site at the reducing end, the entire chain of the saccharide is available to interact with the IgM receptor of the B-cell. This approach allows preparation of terminal saccharides of varying sequences and site-specific addition of other moieties that could enhance the immunogenicity of the conjugates.
The level of IgG antibodies achieved by the O-SP-HSA in mice was lower than those reported for S. dysenteriae type 1 O-SP bound to tetanus toxoid probably because of the greater immunogenicity of the latter protein (14). Minding that the immunogenicity of polysaccharide-based vaccines in mice and monkeys may not always be related to that obtained in human infants for whom these S. dysenteriae type 1 conjugates are targeted, we plan to evaluate clinically these investigational vaccines with medically useful bacterial toxoids as carriers.
Footnotes
Abbreviations. O-SP, O-specific polysaccharide; LPS, lipopolysaccharide; HSA, human serum albumin; EU, ELISA units; MW, molecular weight.
References
- 1.Shiga K. Zentralbl Bakteriol. 1898;24:817–828. [Google Scholar]
- 2.World Health Organisation (1988) WHO/CDD/SER/88.12.
- 3.Raghupathy P, Date A, Shastry J C M, Sudarsanam A, Jadhav M. Brit Med J. 1978;1:1518–1521. doi: 10.1136/bmj.1.6126.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ries A A, Wells J G, Olivola D, Ntakibirora M, Nyandwi S, Ntibakivayo M, Ivey C B, Greene K D, Tenover F C, Wahlquist S P, et al. J Infect Dis. 1994;169:1035–1041. doi: 10.1093/infdis/169.5.1035. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharya S K, Sinha A K, Sengupta P G, Lall P, Pal S C. J Assoc Physicians India. 1988;36:319–320. [PubMed] [Google Scholar]
- 6.Butler T, Islam M R, Azad M A K, Jones P K. J Pediatr. 1987;110:894–897. doi: 10.1016/s0022-3476(87)80405-5. [DOI] [PubMed] [Google Scholar]
- 7.Pal S C, Sengupta P G, Bhattacharya S K, Deb B C. Indian J Med Res. 1989;89:57–64. [PubMed] [Google Scholar]
- 8.0′Brien A, LaVeck G D, Thompson M R, Formal S B. J Infect Dis. 1982;146:763–769. doi: 10.1093/infdis/146.6.763. [DOI] [PubMed] [Google Scholar]
- 9.Ostroff S M, Tarr P I, Neill M A, Lewis J H, Hargrett-Bean N, Kobayashi J M. J Infect Dis. 1989;160:994–998. doi: 10.1093/infdis/160.6.994. [DOI] [PubMed] [Google Scholar]
- 10.Ørskov I, Ørskov F, Jann B, Jann K. Bacteriol Rev. 1977;41:667–710. doi: 10.1128/br.41.3.667-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robbins J B, Chu C, Schneerson R. Clin Infect Dis. 1992;15:346–361. doi: 10.1093/clinids/15.2.346. [DOI] [PubMed] [Google Scholar]
- 12.Kondadu E Y, Parke J C, Jr, Tran H T, Bryla D A, Robbins J B, Szu S C. J Infect Dis. 1998;177:383–387. doi: 10.1086/514203. [DOI] [PubMed] [Google Scholar]
- 13.Robbins J B, Schneerson R, Szu S C. J Infect Dis. 1995;171:1387–1398. doi: 10.1093/infdis/171.6.1387. [DOI] [PubMed] [Google Scholar]
- 14.Chu C, Liu B, Watson D, Szu S, Bryla D, Shiloach J, Schneerson R, Robbins J B. Infect Immun. 1991;59:4450–4458. doi: 10.1128/iai.59.12.4450-4458.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor D N, Trofa A C, Sadoff J, Chu C, Bryla D, Shiloach J, Cohen D, Ashkenazi S, Lerman Y, Egan W, et al. Infect Immun. 1993;61:3678–3687. doi: 10.1128/iai.61.9.3678-3687.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen D, Ashkenazi S, Green M S, Gdalevich M, Robin G, Slepon R, Yavzori M, Orr N, Block C, Ashkenazi I, et al. Lancet. 1997;349:155–159. doi: 10.1016/S0140-6736(96)06255-1. [DOI] [PubMed] [Google Scholar]
- 17.Robbins J B, Schneerson R. J Infect Dis. 1991;161:821–832. doi: 10.1093/infdis/161.5.821. [DOI] [PubMed] [Google Scholar]
- 18.Robbins J B, Schneerson R, Anderson P, Smith D H. J Am Med Assoc. 1996;276:1181–1185. doi: 10.1001/jama.276.14.1181. [DOI] [PubMed] [Google Scholar]
- 19.Dmitriev B A, Knirel N, Kochetkov N K, Hofman I L. Eur J Biochem. 1976;78:381–387. doi: 10.1111/j.1432-1033.1977.tb11750.x. [DOI] [PubMed] [Google Scholar]
- 20.Pavliak V, Nashed J, Pozsgay V, Kovac P, Karpas A, Chu C, Schneerson R, Robbins J B, Glaudemans C P J. J Biol Chem. 1993;268:25797–25802. [PubMed] [Google Scholar]
- 21.Pozsgay V, Glaudemans C P J, Robbins J B, Schneerson R. Tetrahedron. 1992;48:10249–10264. [Google Scholar]
- 22.Pozsgay V, Glaudemans C P J, Robbins J B, Schneerson R. Carbohydr Res. 1993;244:259–273. doi: 10.1016/0008-6215(83)85006-x. [DOI] [PubMed] [Google Scholar]
- 23.Pozsgay V. J Am Chem Soc. 1995;117:6673–6681. [Google Scholar]
- 24.Pozsgay V, Coxon B. Carbohydr Res. 1995;277:171–178. doi: 10.1016/0008-6215(95)00198-3. [DOI] [PubMed] [Google Scholar]
- 25.Pozsgay V. Angew Chem Int Ed Engl. 1998;37:138–141. [Google Scholar]
- 26.Pozsgay V. J Org Chem. 1998;63:5983–5999. doi: 10.1021/jo980660a. [DOI] [PubMed] [Google Scholar]
- 27.Pozsgay V, Sari N, Coxon B. Carbohydr Res. 1998;308:229–238. doi: 10.1016/s0008-6215(98)00047-0. [DOI] [PubMed] [Google Scholar]
- 28.Plikaytis B D, Holder P F, Carlone G M. elisa 12 User’s Manual. Atlanta: Centers for Disease Control; 1995. [Google Scholar]
- 29.Cohen D, Ashkenazi S, Green M, Lerman Y, Slepo N R, Robin G, Orr N, Taylor D N, Sadoff J C, Chu C, et al. Infect Immun. 1996;64:4074–4077. doi: 10.1128/iai.64.10.4074-4077.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen D, Green M S, Block C, Rouach T, Ofek I. J Infect Dis. 1988;157:1068–1071. doi: 10.1093/infdis/157.5.1068. [DOI] [PubMed] [Google Scholar]
- 31.Peeters C A M, Evenberg D, Hoogerhout P, Käyhty H, Saarinen H L, van Boeckel C A, van der Marel G A, van Boom J H, Poolman J T. Infect Immun. 1992;60:1826–1833. doi: 10.1128/iai.60.5.1826-1833.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson P, Pichichero M E, Insel R A. J Clin Invest. 1985;76:52–59. doi: 10.1172/JCI111976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kabat E A, Bezer A E. Arch Biochem Biophys. 1958;8:306–310. doi: 10.1016/0003-9861(58)90354-0. [DOI] [PubMed] [Google Scholar]
- 34.Lindberg A A, Rosenberg L T, Lungren A, Garegg P J, Svensson S, Wallin N H. Infect Immun. 1974;10:541–545. doi: 10.1128/iai.10.3.541-545.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gotschlich E C, Rey M, Sanborn W R, Triau R, Cvjetanovic B. Prog Immunobiol Stand. 1972;129:485–491. [PubMed] [Google Scholar]
- 36.Szu S C, Li X, Schneerson R, Vickers J, Robbins J B. Infect Immun. 1989;57:3823–3827. doi: 10.1128/iai.57.12.3823-3827.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson P W, Pichichero M E, Stein E C, Porcalli S, Betts R F, Connuck D M, Lorones D, Insel R A, Zahradnik J M, Eby R. J Immunol. 1989;142:2464–2468. [PubMed] [Google Scholar]
- 38.Dintzis R Z, Okajima M, Middleton M H, Greene G, Dintzis H M. J Immunol. 1989;143:1239–1244. [PubMed] [Google Scholar]
- 39.Laferriére C A, Sood R K, de Muys J-M, Michon F, Jennings H J. Vaccine. 1997;15:179–186. doi: 10.1016/s0264-410x(96)00148-x. [DOI] [PubMed] [Google Scholar]
- 40.Kupfer A, Swain S L, Janeway C A, Singer S J. Proc Natl Acad Sci USA. 1986;83:6080–6083. doi: 10.1073/pnas.83.16.6080. [DOI] [PMC free article] [PubMed] [Google Scholar]