Abstract
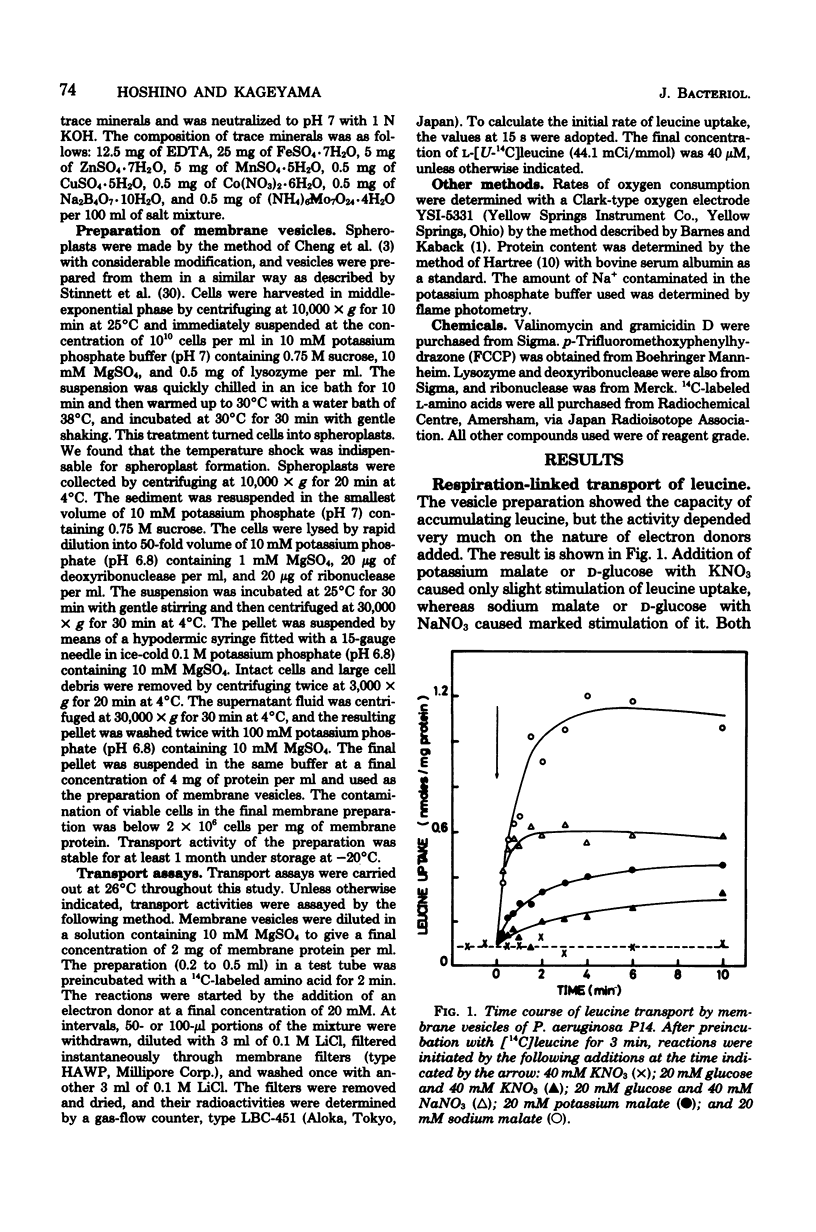
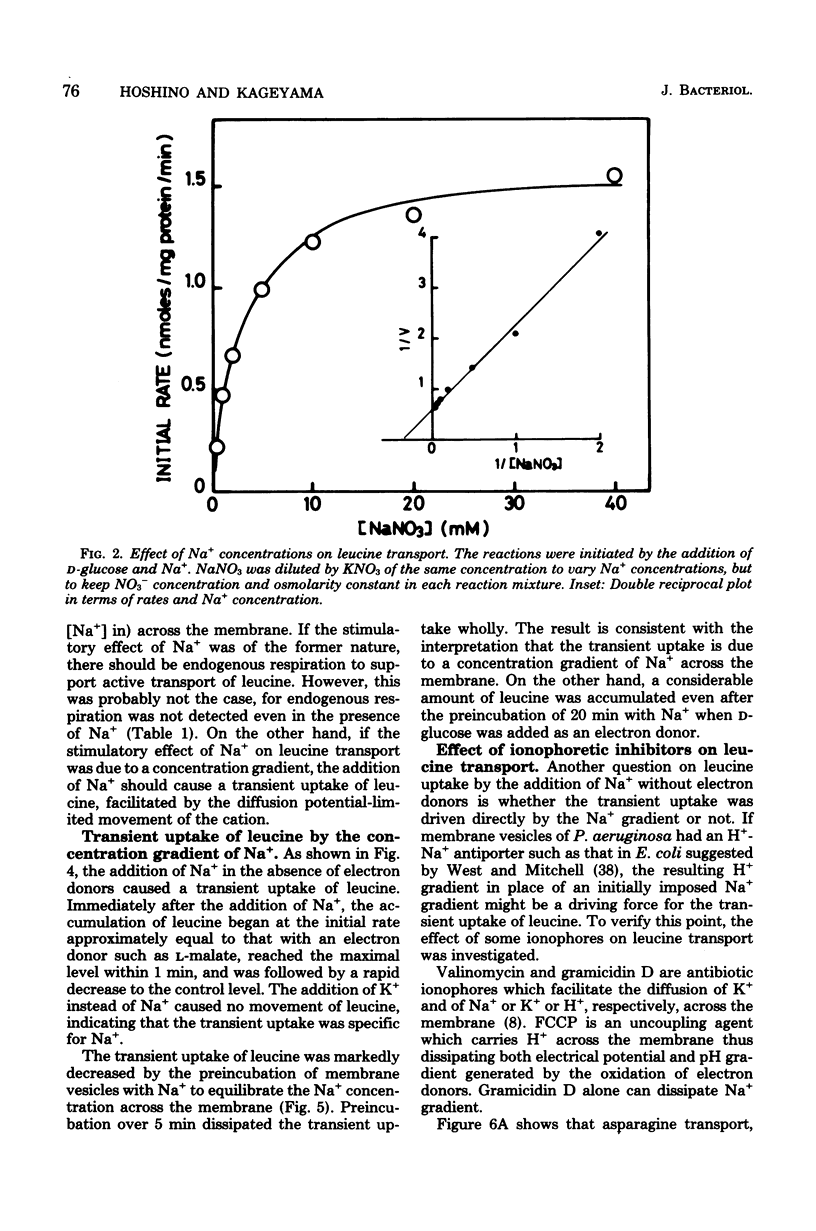
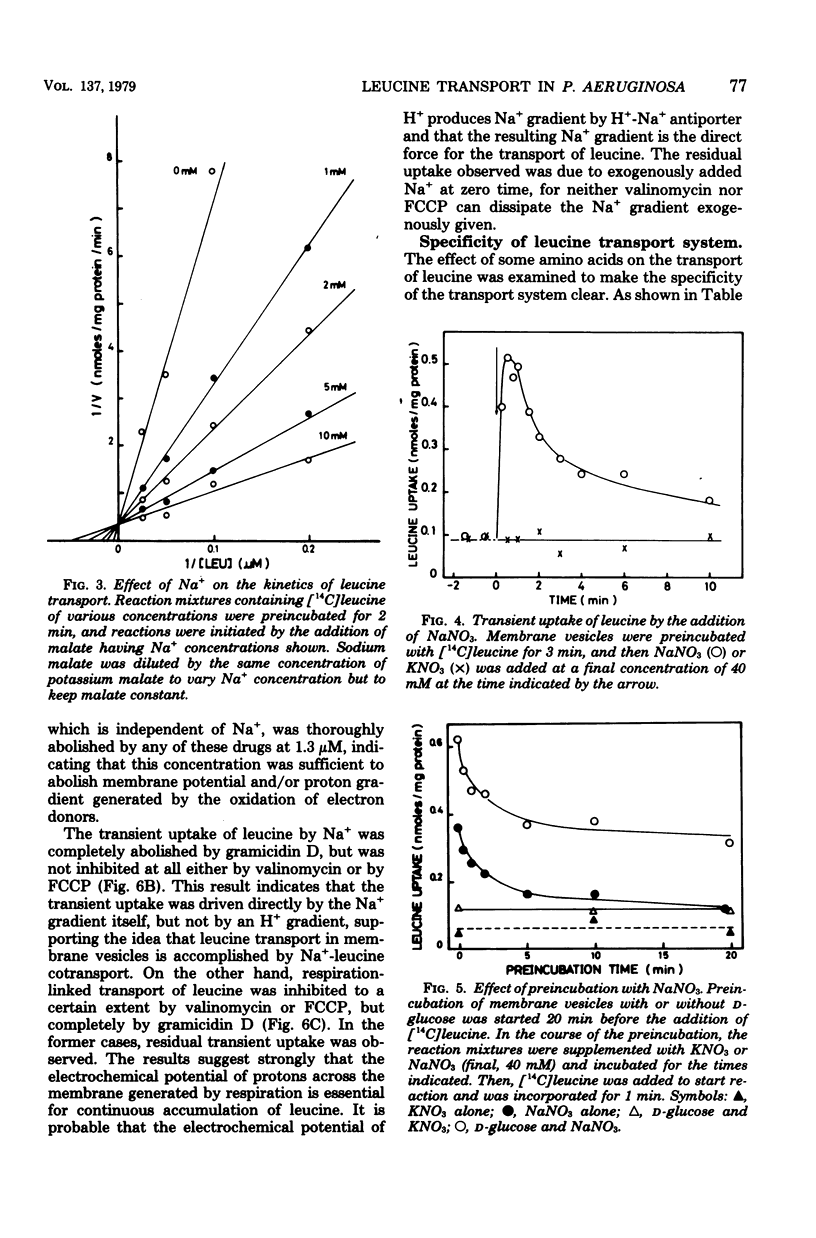
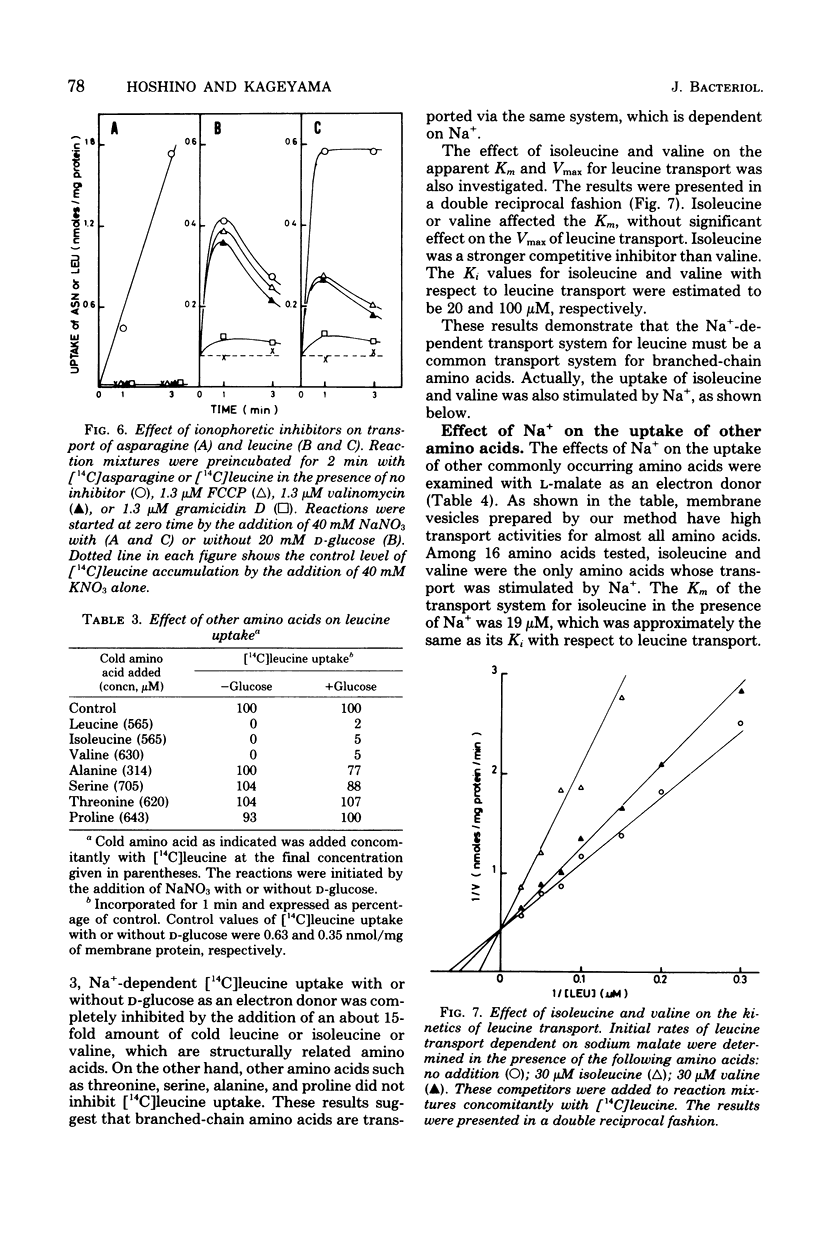
Membrane vesicles were prepared by osmotic lysis of spheroplasts of Pseudomonas aeruginosa strain P14, and the active transport of amino acids was studied. D-Glucose, gluconate, and L-malate supported active transport of various L-amino acids. The respiration-dependent leucine transport was markedly stimulated by Na+. Moreover, without any respiratory substrate, leucine was also transported transiently by the addition of Na+ alone. This transient uptake of leucine was not inhibited either by carbonyl cyanide p-trifluoromethyoxyphenylhydrazone or by valinomycin, but was completely abolished by gramicidin D. Increase in the concentration of Na+ of the medium resulted in a decrease of the Km for L-leucine transport, whereas the Vmax was not significnatly affected. Active transport of leucine was inhibited competitively by isoleucine or by valine, whose transport was also stimulated by Na+. On the other hand, Na+ was not required for the uptake of other L-amino acids tested, but rather was inhibitory for some of them. These results show (i) that a common transport system for branched-chain amino acids exists in membrane vesicles, (ii) that the system requires Na+ for its activity, and (iii) that an Na+ gradient can drive the system.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes E. M., Jr, Kaback H. R. Mechanisms of active transport in isolated membrane vesicles. I. The site of energy coupling between D-lactic dehydrogenase and beta-galactoside transport in Escherichia coli membrane vesicles. J Biol Chem. 1971 Sep 10;246(17):5518–5522. [PubMed] [Google Scholar]
- CAMPBELL J. J., HOGGLA, STRASDINE G. A. Enzyme distribution in Pseudomonas aeruginosa. J Bacteriol. 1962 May;83:1155–1160. doi: 10.1128/jb.83.5.1155-1160.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. J., Ingram J. M., Costerton J. W. Interactions of alkaline phosphatase and the cell wall of Pseudomonas aeruginosa. J Bacteriol. 1971 Jul;107(1):325–336. doi: 10.1128/jb.107.1.325-336.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane R. K. Na+ -dependent transport in the intestine and other animal tissues. Fed Proc. 1965 Sep-Oct;24(5):1000–1006. [PubMed] [Google Scholar]
- Frank L., Hopkins I. Sodium-stimulated transport of glutamate in Escherichia coli. J Bacteriol. 1969 Oct;100(1):329–336. doi: 10.1128/jb.100.1.329-336.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guymon L. F., Eagon R. G. Transport of glucose, gluconate, and methyl alpha-D-glucoside by Pseudomonas aeruginosa. J Bacteriol. 1974 Mar;117(3):1261–1269. doi: 10.1128/jb.117.3.1261-1269.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern Y. S., Barash H., Dover S., Druck K. Sodium and potassium requirements for active transport of glutamate by Escherichia coli K-12. J Bacteriol. 1973 Apr;114(1):53–58. doi: 10.1128/jb.114.1.53-58.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Hasan S. M., Tsuchiya T. Glutamate transport driven by an electrochemical gradient of sodium ion in membrane vesicles of Escherichia coli B. Biochem Biophys Res Commun. 1977 Sep 9;78(1):122–128. doi: 10.1016/0006-291x(77)91229-3. [DOI] [PubMed] [Google Scholar]
- Hirata H., Kosmakos F. C., Brodie A. F. Active transport of proline in membrane preparations from Mycobacterium phlei. J Biol Chem. 1974 Nov 10;249(21):6965–6970. [PubMed] [Google Scholar]
- Kahane S., Marcus M., Barash H., Halpern Y. S. Sodium-dependent glutamate transport in membrane vesicles of Escherichia coli K-12. FEBS Lett. 1975 Aug 15;56(2):235–239. doi: 10.1016/0014-5793(75)81099-4. [DOI] [PubMed] [Google Scholar]
- Kay W. W., Gronlund A. F. Amino acid pool formation in Pseudomonas aeruginosa. J Bacteriol. 1969 Jan;97(1):282–291. doi: 10.1128/jb.97.1.282-291.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay W. W., Gronlund A. F. Amino acid transport in Pseudomonas aeruginosa. J Bacteriol. 1969 Jan;97(1):273–281. doi: 10.1128/jb.97.1.273-281.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay W. W., Gronlund A. F. Influence of carbon or nitrogen starvation on amino acid transport in Pseudomonas aeruginosa. J Bacteriol. 1969 Oct;100(1):276–282. doi: 10.1128/jb.100.1.276-282.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay W. W., Gronlund A. F. Proline transport by Pseudomonas aeruginosa. Biochim Biophys Acta. 1969;193(2):444–455. doi: 10.1016/0005-2736(69)90203-x. [DOI] [PubMed] [Google Scholar]
- Kay W. W., Gronlund A. F. Transport of aromatic amino acids by Pseudomonas aeruginosa. J Bacteriol. 1971 Mar;105(3):1039–1046. doi: 10.1128/jb.105.3.1039-1046.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanyi J. K., Renthal R., MacDonald R. E. Light-induced glutamate transport in Halobacterium halobium envelope vesicles. II. Evidence that the driving force is a light-dependent sodium gradient. Biochemistry. 1976 Apr 20;15(8):1603–1610. doi: 10.1021/bi00653a002. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K., Yearwood-Drayton V., MacDonald R. E. Light-induced glutamate transport in Halobacterium halobium envelope vesicles. I. Kinetics of the light-dependent and the sodium-gradient-dependent uptake. Biochemistry. 1976 Apr 20;15(8):1595–1603. doi: 10.1021/bi00653a001. [DOI] [PubMed] [Google Scholar]
- Lombardi F. J., Reeves J. P., Short S. A., Kaback H. R. Evaluation of the chemiosmotic interpretation of active transport in bacterial membrane vesicles. Ann N Y Acad Sci. 1974 Feb 18;227:312–327. doi: 10.1111/j.1749-6632.1974.tb14396.x. [DOI] [PubMed] [Google Scholar]
- Lopilato J., Tsuchiya T., Wilson T. H. Role of Na+ and Li+ in thiomethylgalactoside transport by the melibiose transport system of Escherichia coli. J Bacteriol. 1978 Apr;134(1):147–156. doi: 10.1128/jb.134.1.147-156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald R. E., Lanyi J. K., Greene R. V. Sodium-stimulated glutamate uptake in membrane vesicles of Escherichia coli: the role of ion gradients. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3167–3170. doi: 10.1073/pnas.74.8.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald R. E., Lanyi L. K. Light-induced leucine transport in Halobacterium halobium envelope vesicles: a chemiosmotic system. Biochemistry. 1975 Jul;14(13):2882–2889. doi: 10.1021/bi00684a014. [DOI] [PubMed] [Google Scholar]
- Midgley M., Dawes E. A. The regulation of transport of glucose and methyl alpha-glucoside in Pseudomonas aeruginosa. Biochem J. 1973 Feb;132(2):141–154. doi: 10.1042/bj1320141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner K. M., Frank L. Sodium-stimulated glutamate transport in osmotically shocked cells and membrane vesicles of Escherichia coli. J Bacteriol. 1974 Mar;117(3):1093–1098. doi: 10.1128/jb.117.3.1093-1098.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Ramos S., Schuldiner S., Kaback H. R. The electrochemical gradient of protons and its relationship to active transport in Escherichia coli membrane vesicles. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1892–1896. doi: 10.1073/pnas.73.6.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S. G., Curran P. F. Coupled transport of sodium and organic solutes. Physiol Rev. 1970 Oct;50(4):637–718. doi: 10.1152/physrev.1970.50.4.637. [DOI] [PubMed] [Google Scholar]
- Stinnett J. D., Guymon L. F., Eagon R. G. A novel technique for the preparation of transport-active membrane vesicles from Pseudomonas aeruginosa: observations on gluconate transport. Biochem Biophys Res Commun. 1973 May 1;52(1):285–290. doi: 10.1016/0006-291x(73)90985-6. [DOI] [PubMed] [Google Scholar]
- Stock J., Roseman S. A sodium-dependent sugar co-transport system in bacteria. Biochem Biophys Res Commun. 1971 Jul 2;44(1):132–138. doi: 10.1016/s0006-291x(71)80168-7. [DOI] [PubMed] [Google Scholar]
- Thompson J., MacLeod R. A. Functions of Na+ and K+ in the active transport of -aminoisobutyric acid in a marine pseudomonad. J Biol Chem. 1971 Jun 25;246(12):4066–4074. [PubMed] [Google Scholar]
- Tiwari N. P., Campbell J. J. Utilization of dicarboxylic acids by Pseudomonas aeruginosa. Can J Microbiol. 1969 Sep;15(9):1095–1100. doi: 10.1139/m69-194. [DOI] [PubMed] [Google Scholar]
- Tokuda H., Kaback H. R. Sodium-dependent binding of p-nitrophenyl alpha-D-galactopyranoside to membrane vesicles isolated from Salmonella typhimurium. Biochemistry. 1978 Feb 21;17(4):698–705. doi: 10.1021/bi00597a022. [DOI] [PubMed] [Google Scholar]
- Tokuda H., Kaback H. R. Sodium-dependent methyl 1-thio-beta-D-galactopyranoside transport in membrane vesicles isolated from Salmonella typhimurium. Biochemistry. 1977 May 17;16(10):2130–2136. doi: 10.1021/bi00629a013. [DOI] [PubMed] [Google Scholar]
- Tsuchiya T., Hasan S. M., Raven J. Glutamate transport driven by an electrochemical gradient of sodium ions in Escherichia coli. J Bacteriol. 1977 Sep;131(3):848–853. doi: 10.1128/jb.131.3.848-853.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya T., Raven J., Wilson T. H. Co-transport of Na+ and methul-beta-D-thiogalactopyranoside mediated by the melibiose transport system of Escherichia coli. Biochem Biophys Res Commun. 1977 May 9;76(1):26–31. doi: 10.1016/0006-291x(77)91663-1. [DOI] [PubMed] [Google Scholar]
- West I. C., Mitchell P. Proton/sodium ion antiport in Escherichia coli. Biochem J. 1974 Oct;144(1):87–90. doi: 10.1042/bj1440087. [DOI] [PMC free article] [PubMed] [Google Scholar]



