Abstract
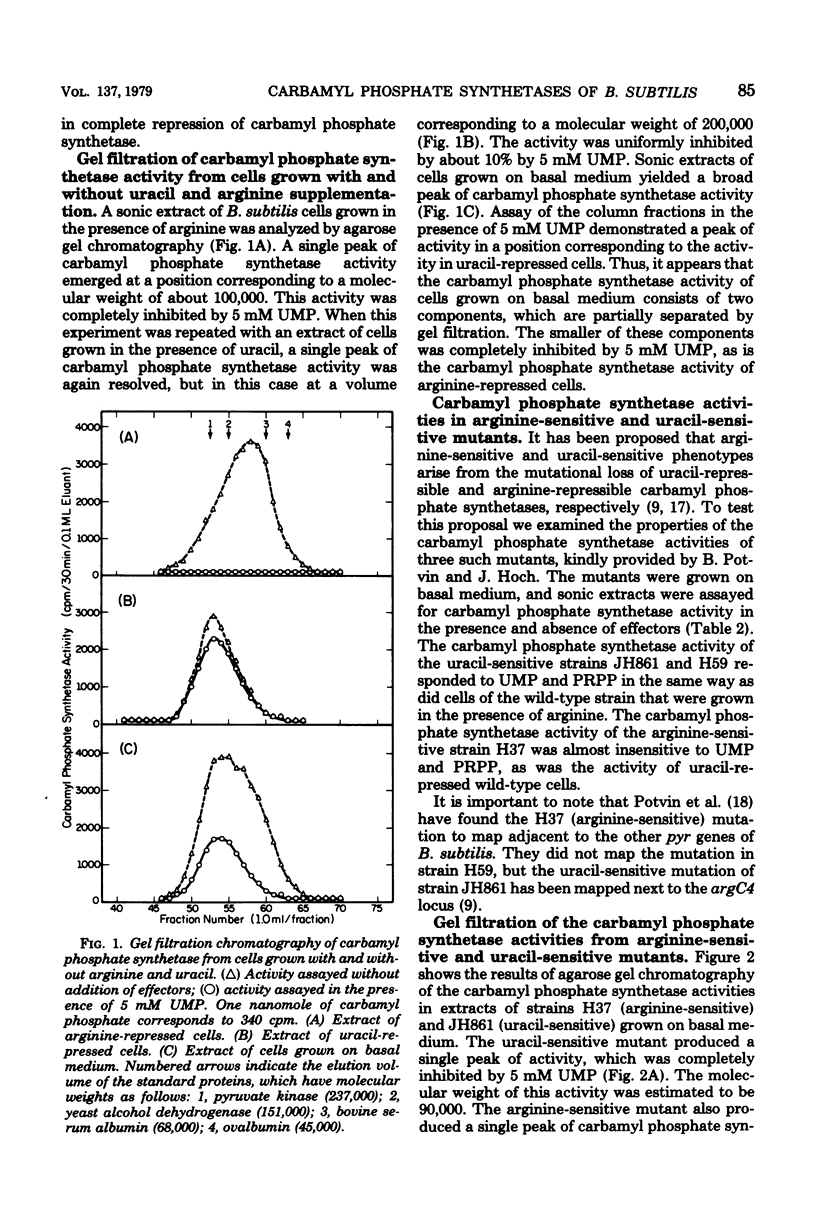
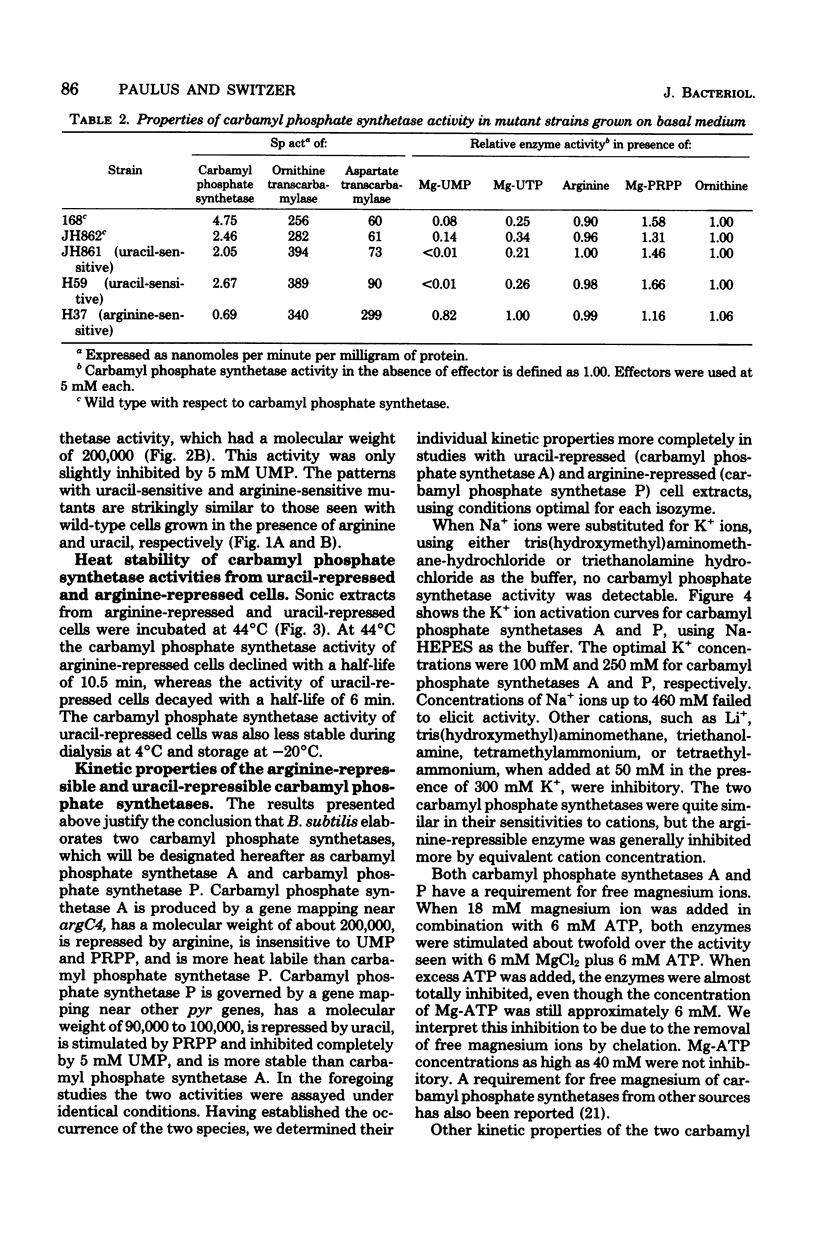
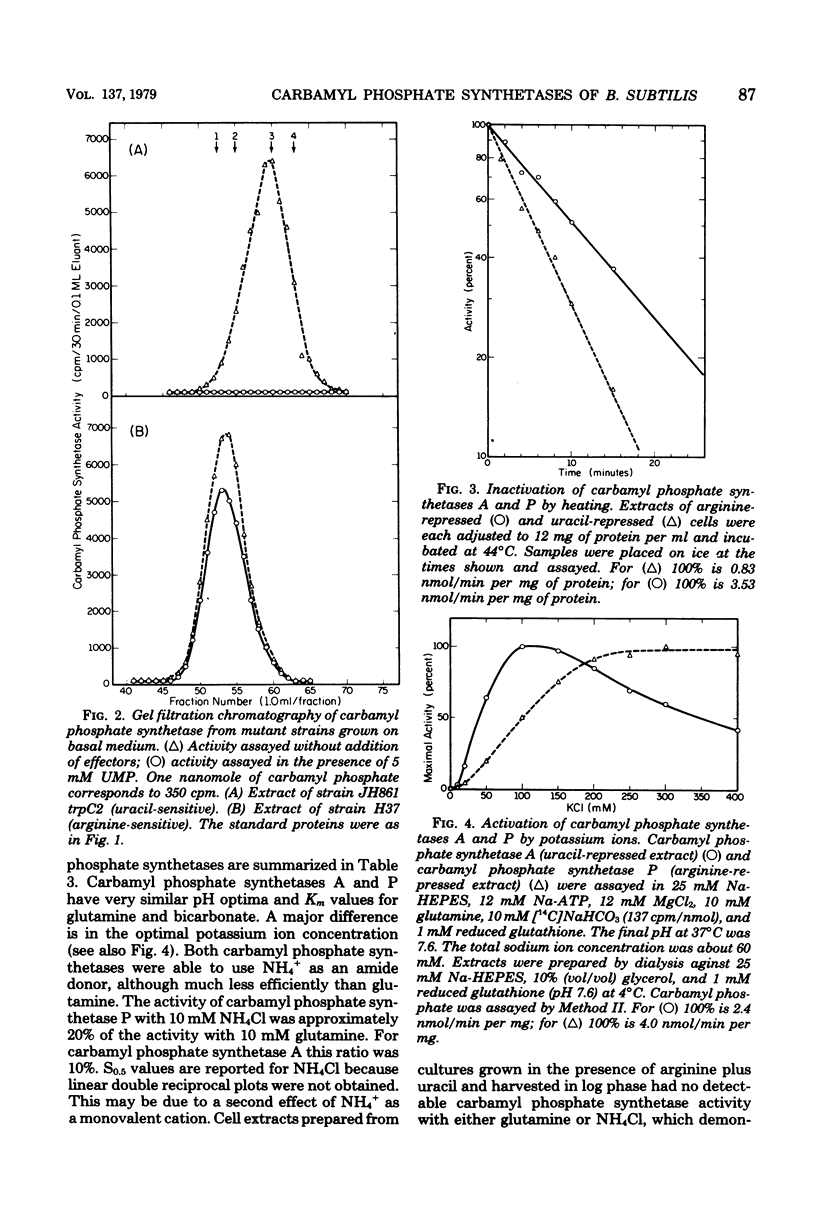
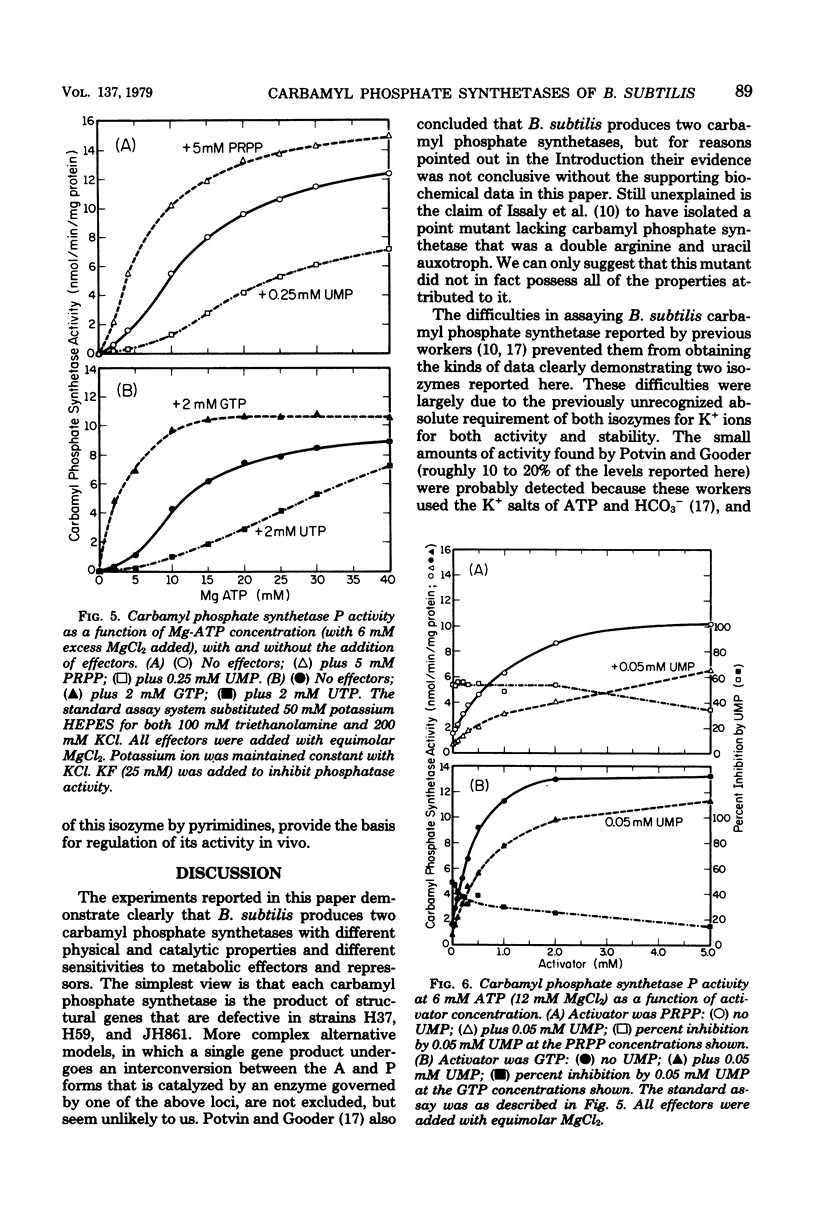
The number and properties of carbamyl phosphate synthetases in Bacillus subtilis have been uncertain because of conflicting genetic results and instability of the enzyme in extracts. The discovery of a previously unrecognized requirement of B. subtilis carbamyl phosphate synthetases for a high concentration of potassium ions for activity and stability permitted unequivocal demonstration that this bacterium elaborates two carbamyl phosphate synthetases. Carbamyl phosphate synthetase A was shown to be repressed by arginine, to have a molecular weight of about 200,000, and to be coded for by a gene that maps near argC4. This isozyme was insensitive to metabolites of the arginine and pyrimidine biosynthetic pathways. Carbamyl phosphate synthetase P was found to be repressed by uracil, to have a molecular weight of 90,000 to 100,000, and to be coded for by a gene that maps near the other pyr genes. This isozyme was activated by phosphoridine nucleotides. Other kinetic properties of the two isozymes were compared. Bacillus thus resembles eucaryotic microbes in producing two carbamyl phosphate synthetases, rather than the enteric bacteria, which produce a single carbamyl phosphate synthetase.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abd-el-Al A., Ingraham J. L. Control of carbamyl phosphate synthesis in Salmonella typhimurium. J Biol Chem. 1969 Aug 10;244(15):4033–4038. [PubMed] [Google Scholar]
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabson J. S., Switzer R. L. Purification and properties of Bacillus subtilis aspartate transcarbamylase. J Biol Chem. 1975 Nov 25;250(22):8664–8669. [PubMed] [Google Scholar]
- Cox D. P., Hanson R. S. Catabolite repression of aconitate hydratase in Bacillus subtilis. Biochim Biophys Acta. 1968 Apr 16;158(1):36–44. doi: 10.1016/0304-4165(68)90069-x. [DOI] [PubMed] [Google Scholar]
- Eisenstadt E. Potassium content during growth and sporulation in Bacillus subtilis. J Bacteriol. 1972 Oct;112(1):264–267. doi: 10.1128/jb.112.1.264-267.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann E. C., Dunn J. H., Schmidt R. R. DEAE paper chromatography to separate intermediates of the pyrimidine biosynthetic pathway and to assay asparatate transcarbamylase and dihydroorotase activities. Anal Biochem. 1973 Jun;53(2):478–483. doi: 10.1016/0003-2697(73)90097-3. [DOI] [PubMed] [Google Scholar]
- Issaly I. M., Issaly A. S., Reissig J. L. Carbamyl phosphate biosynthesis in Bacillus subtilis. Biochim Biophys Acta. 1970 Mar 18;198(3):482–494. doi: 10.1016/0005-2744(70)90126-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lacroute F., Piérard A., Grenson M., Wiame J. M. The biosynthesis of carbamoyl phosphate in Saccharomyces cerevisiae. J Gen Microbiol. 1965 Jul;40(1):127–142. doi: 10.1099/00221287-40-1-127. [DOI] [PubMed] [Google Scholar]
- Legrain C., Stalon V., Noullez J. P., Mercenier A., Simon J. P., Broman K., Wiame J. M. Structure and function of ornithine carbamoyltransferases. Eur J Biochem. 1977 Nov 1;80(2):401–409. doi: 10.1111/j.1432-1033.1977.tb11895.x. [DOI] [PubMed] [Google Scholar]
- Levine R. L., Kretchmer N. Conversion of carbamoyl phosphate to hydroxyurea. An assay for carbamoylphosphate synthetase. Anal Biochem. 1971 Aug;42(2):324–337. doi: 10.1016/0003-2697(71)90044-3. [DOI] [PubMed] [Google Scholar]
- NEUMANN J., JONES M. E. END-PRODUCT INHIBITION OF ASPARTATE TRANSCARBAMYLASE IN VARIOUS SPECIES. Arch Biochem Biophys. 1964 Mar;104:438–447. doi: 10.1016/0003-9861(64)90487-4. [DOI] [PubMed] [Google Scholar]
- O'Donovan G. A., Neuhard J. Pyrimidine metabolism in microorganisms. Bacteriol Rev. 1970 Sep;34(3):278–343. doi: 10.1128/br.34.3.278-343.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvin B. W., Kelleher R. J., Jr, Gooder H. Pyrimidine biosynthetic pathway of Baccillus subtilis. J Bacteriol. 1975 Aug;123(2):604–615. doi: 10.1128/jb.123.2.604-615.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvin B., Gooder H. Carbamyl phosphate synthesis in Bacillus subtilis. Biochem Genet. 1975 Feb;13(1-2):125–143. doi: 10.1007/BF00486011. [DOI] [PubMed] [Google Scholar]
- Prescott L. M., Jones M. E. Modified methods for the determination of carbamyl aspartate. Anal Biochem. 1969 Dec;32(3):408–419. doi: 10.1016/s0003-2697(69)80008-4. [DOI] [PubMed] [Google Scholar]
- Tatibana M., Shigesada K. Control of pyrimidine biosynthesis in mammalian tissues. IV. Requirements of a quantitative assay of glutamine-dependent carbamyl phosphate synthetase and effect of magnesium ion as an essential activator. J Biochem. 1972 Sep;72(3):537–547. doi: 10.1093/oxfordjournals.jbchem.a129933. [DOI] [PubMed] [Google Scholar]
- Tatibana M., Shigesada K. Control of pyrimidine biosynthesis in mammalian tissues. V. Regulation of glutamine-dependent carbamyl phosphate synthetase: activation by 5-phosphoribosyl 1-pyrophosphate and inhibition by uridine triphosphate. J Biochem. 1972 Sep;72(3):549–560. doi: 10.1093/oxfordjournals.jbchem.a129934. [DOI] [PubMed] [Google Scholar]
- Williams L. G., Bernhardt S. A., Davis R. H. Evidence for two discrete carbamyl phosphate pools in Neurospora. J Biol Chem. 1971 Feb 25;246(4):973–978. [PubMed] [Google Scholar]
- Williams L. G., Davis R. H. Pyrimidine-specific carbamyl phosphate synthetase in Neurospora crassa. J Bacteriol. 1970 Aug;103(2):335–341. doi: 10.1128/jb.103.2.335-341.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


