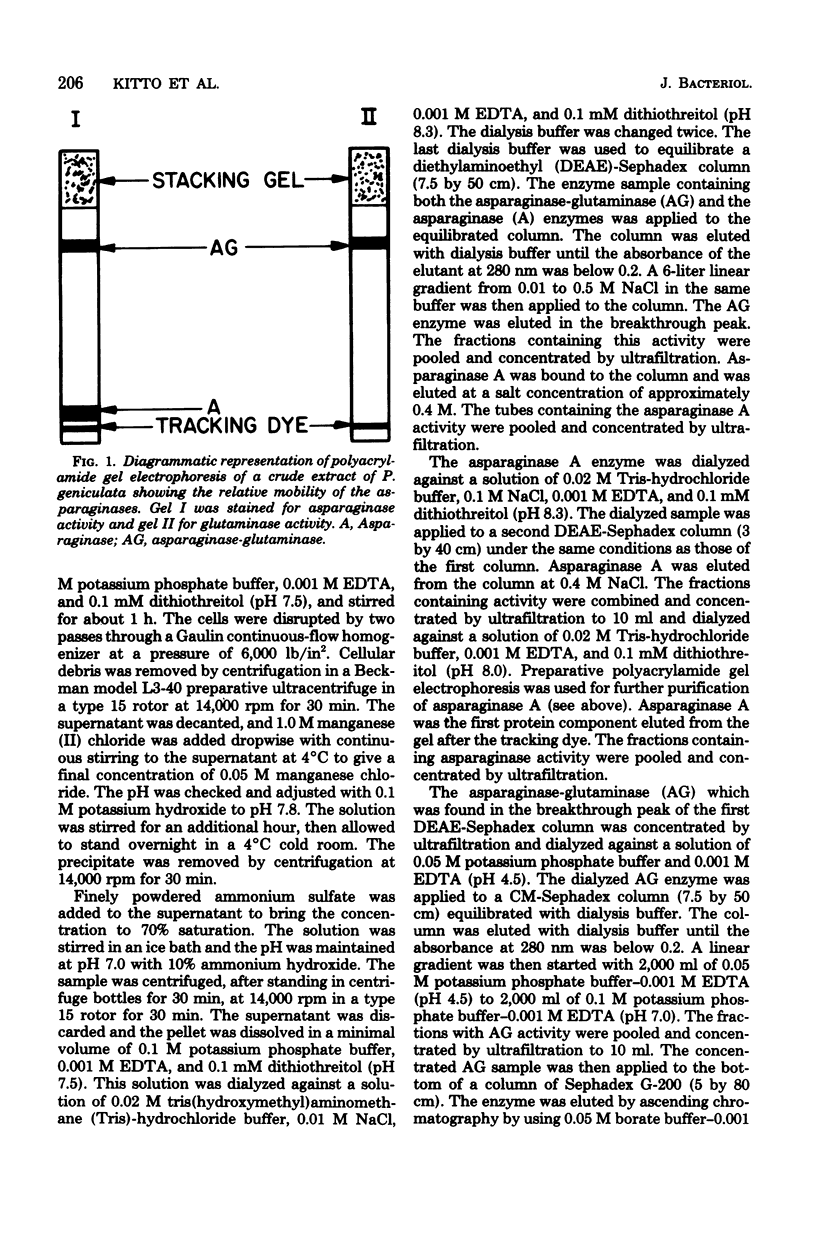
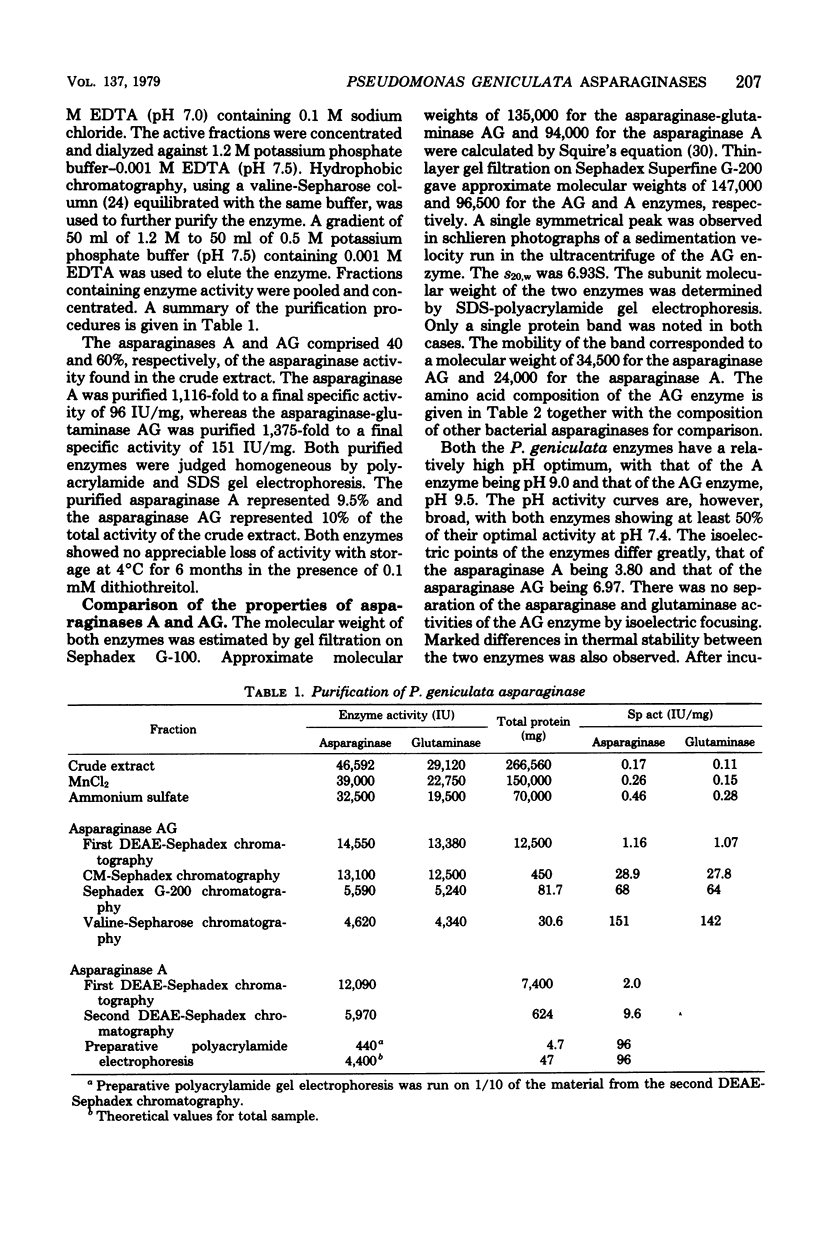
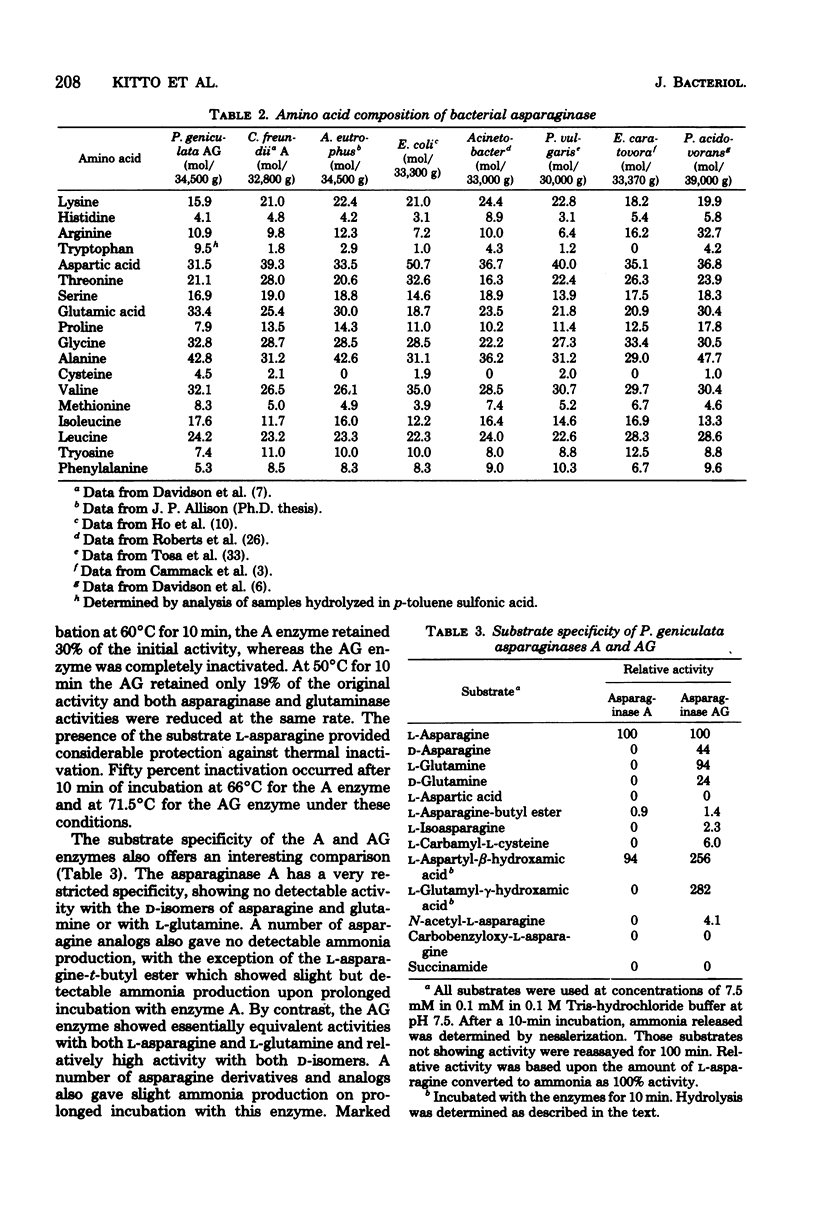
Abstract
Two enzymes that catalyze the hydrolysis of l-asparagine have been isolated from extracts of Pseudomonas geniculata. After initial salt fractionation, the enzymes were separated by chromatography on diethylaminoethyl-Sephadex and purified to homogeneity by gel filtration, ion-exchange chromatography, and preparative polyacrylamide electrophoresis. The enzymes differ markedly in physicochemical properties. One enzyme, termed asparaginase A, has a molecular weight of approximately 96,000 whereas the other, termed asparaginase AG, has a molecular weight of approximately 135,000. Both enzymes are tetrameric. The asparaginase A shows activity only with l-asparagine as substrate, whereas the asparaginase AG hydrolyzes l-asparagine and l-glutamine at approximately equal rates and it is also active with d-asparagine and d-glutamine as substrates. The asparaginase A was found to be devoid of antitumor activity in mice, whereas the asparaginase AG was effective in increasing the mean survival times of both C3H mice carrying the asparagine-requiring Gardner 6C3HED tumor line and Swiss mice bearing the glutamine-requiring Ehrlich ascites tumor line. These differences in antitumor activity were related to differences in the Km values for l-asparagine for the two enzymes. The asparaginase A has a Km value of 1 × 10−3 M for this substrate whereas the corresponding value for the AG enzyme is 1.5 × 10−5 M. Thus the concentration of asparagine necessary for maximal activity of the asparaginase A is very high compared with that of the normal plasma level of asparagine, which is approximately 50 μM.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burchenal J. H., Karnofsky D. A. Clinical evaluation of L-asparaginase. Introduction. Cancer. 1970 Feb;25(2):241–243. doi: 10.1002/1097-0142(197002)25:2<241::aid-cncr2820250202>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Cooney D. A., Moore A. L., Zhagats R. A colorimetric technique for the demonstration of L-asparaginase in electropherograms. Clin Chim Acta. 1972 Aug;40(1):249–257. doi: 10.1016/0009-8981(72)90278-1. [DOI] [PubMed] [Google Scholar]
- Crowther D. L-asparaginase and human malignant disease. Nature. 1971 Jan 15;229(5281):168–171. doi: 10.1038/229168a0. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Davidson L., Brear D. R., Wingard P., Hawkins J., Kitto G. B. Purification and properties of L-glutaminase-L-asparaginase from Pseudomonas acidovorans. J Bacteriol. 1977 Mar;129(3):1379–1386. doi: 10.1128/jb.129.3.1379-1386.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson L., Burkom M., Ahn S., Chang L. C., Kitto B. L-Asparagainases from Citrobacter freundii. Biochim Biophys Acta. 1977 Jan 11;480(1):282–294. doi: 10.1016/0005-2744(77)90341-2. [DOI] [PubMed] [Google Scholar]
- Goldberg A. I., Cooney D. A., Glynn J. P., Homan E. R., Gaston M. R., Milman H. A. The effects of immunization to L-asparaginase on antitumor and enzymatic activity. Cancer Res. 1973 Feb;33(2):256–261. [PubMed] [Google Scholar]
- Ho P. P., Milikin E. B., Bobbitt J. L., Grinnan E. L., Burck P. J., Frank B. H., Boeck L. D., Squires R. W. Crystalline L-asparaginase from Escherichia coli B. I. Purification and chemical characterization. J Biol Chem. 1970 Jul 25;245(14):3708–3715. [PubMed] [Google Scholar]
- Holcenberg J. S., Teller D. C., Roberts J., Dolowy W. C. Physical properties of Acinetobacter glutaminase-asparaginase with antitumor activity. J Biol Chem. 1972 Dec 10;247(23):7750–7758. [PubMed] [Google Scholar]
- Jayaram H. N., Ramakrishnan R., Vaidyanathan C. S. L-asparaginases from Mycobacterium tuberculosis strains H37Rv and H37Ra. Arch Biochem Biophys. 1968 Jul;126(1):165–174. doi: 10.1016/0003-9861(68)90570-5. [DOI] [PubMed] [Google Scholar]
- Katsumata H., Katsumata R., Abe T., Takenaka O., Inada Y. Purification and physicochemical properties of L-glutaminase from Pseudomonas. Biochim Biophys Acta. 1972 Dec 7;289(2):405–409. doi: 10.1016/0005-2744(72)90093-9. [DOI] [PubMed] [Google Scholar]
- King O. Y., Wilbur J. R., Mumford D. M., Sutow W. W. Therapy with Erwinia L-asparaginase in children with acute leukemia after anaphylaxis to E. coli L-asparaginase. Cancer. 1974 Mar;33(3):611–614. doi: 10.1002/1097-0142(197403)33:3<611::aid-cncr2820330303>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- McCredie K. B., Ho D. H., Freireich E. J. L-asparaginase for the treatment of cancer. CA Cancer J Clin. 1973 Jul-Aug;23(4):220–227. doi: 10.3322/canjclin.23.4.220. [DOI] [PubMed] [Google Scholar]
- Oettgen H. F., Stephenson P. A., Schwartz M. K., Leeper R. D., Tallai L., Tan C. C., Clarkson B. D., Golbey R. B., Krakoff I. H., Karnofsky D. A. Toxicity of E. coli L-asparaginase in man. Cancer. 1970 Feb;25(2):253–278. doi: 10.1002/1097-0142(197002)25:2<253::aid-cncr2820250204>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Peterson R. E., Ciegler A. L-asparaginase production by various bacteria. Appl Microbiol. 1969 Jun;17(6):929–930. doi: 10.1128/am.17.6.929-930.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMADANM EL-D, ELASMAR F., GREENBERG D. M. PURIFICATION AND PROPERTIES OF GLUTAMINASE AND ASPARAGINASE FROM A PSEUDOMONAD. I. PURIFICATION AND PHYSICAL CHEMICAL PROPERTIES. Arch Biochem Biophys. 1964 Oct;108:143–149. doi: 10.1016/0003-9861(64)90365-0. [DOI] [PubMed] [Google Scholar]
- Riley V., Campbell H. A., Stock C. C. Asparaginase clearance: influence of the LDH-elevating virus. Proc Soc Exp Biol Med. 1970 Jan;133(1):38–42. doi: 10.3181/00379727-133-34402. [DOI] [PubMed] [Google Scholar]
- Riley V., Spackman D., Fitzmaurice M. A., Roberts J., Holcenberg J. S., Dolowy W. C. Therapeutic properties of a new glutaminase-asparaginase preparation and the influence of the lactate dehydrogenase-elevating virus. Cancer Res. 1974 Feb;34(2):429–438. [PubMed] [Google Scholar]
- Rimerman R. A., Hatfield G. W. Phosphate-induced protein chromatography. Science. 1973 Dec 21;182(4118):1268–1270. doi: 10.1126/science.182.4118.1268. [DOI] [PubMed] [Google Scholar]
- Roberts J., Holcenberg J. S., Dolowy W. C. Antineoplastic activity of highly purified bacterial glutaminases. Nature. 1970 Sep 12;227(5263):1136–1137. doi: 10.1038/2271136a0. [DOI] [PubMed] [Google Scholar]
- Roberts J., Holcenberg J. S., Dolowy W. C. Isolation, crystallization, and properties of Achromobacteraceae glutaminase-asparaginase with antitumor activity. J Biol Chem. 1972 Jan 10;247(1):84–90. [PubMed] [Google Scholar]
- Roberts J., Prager M. D., Bachynsky N. The antitumor activity of Escherichia coli L-asparaginase. Cancer Res. 1966 Oct;26(10):2213–2217. [PubMed] [Google Scholar]
- SQUIRE P. G. A RELATIONSHIP BETWEEN THE MOLECULAR WEIGHTS OF MACROMOLECULES AND THEIR ELUTION VOLUMES BASED ON A MODEL FOR SEPHADEX GEL FILTRATION. Arch Biochem Biophys. 1964 Sep;107:471–478. doi: 10.1016/0003-9861(64)90303-0. [DOI] [PubMed] [Google Scholar]
- Shifrin S., Solis B. G., Chalken I. M. L-Asparaginase from Erwinia carotovora. Physicochemical properties of the native and succinylated enzyme. J Biol Chem. 1973 May 25;248(10):3464–3469. [PubMed] [Google Scholar]
- Soda K., Oshima M., Yamamoto T. Purification and properties of isozymes of glutaminase from Pseudomonas aeruginosa. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1278–1284. doi: 10.1016/s0006-291x(72)80113-x. [DOI] [PubMed] [Google Scholar]
- Tallal L., Tan C., Oettgen H., Wollner N., McCarthy M., Helson L., Burchenal J., Karnofsky D., Murphy M. L. E. coli L-asparaginase in the treatment of leukemia and solid tumors in 131 children. Cancer. 1970 Feb;25(2):306–320. doi: 10.1002/1097-0142(197002)25:2<306::aid-cncr2820250206>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Tosa T., Sano R., Yamamoto K., Nakamura M., Chibata I. L-asparaginase from Proteus vulgaris. Purification, crystallization, and enzymic properties. Biochemistry. 1972 Jan 18;11(2):217–222. doi: 10.1021/bi00752a012. [DOI] [PubMed] [Google Scholar]
- Whelan H. A., Wriston J. C., Jr Purification and properties of asparaginase from escherichia coli B. Biochemistry. 1969 Jun;8(6):2386–2393. doi: 10.1021/bi00834a020. [DOI] [PubMed] [Google Scholar]
- Wriston J. C., Jr, Yellin T. O. L-asparaginase: a review. Adv Enzymol Relat Areas Mol Biol. 1973;39:185–248. doi: 10.1002/9780470122846.ch3. [DOI] [PubMed] [Google Scholar]