Abstract
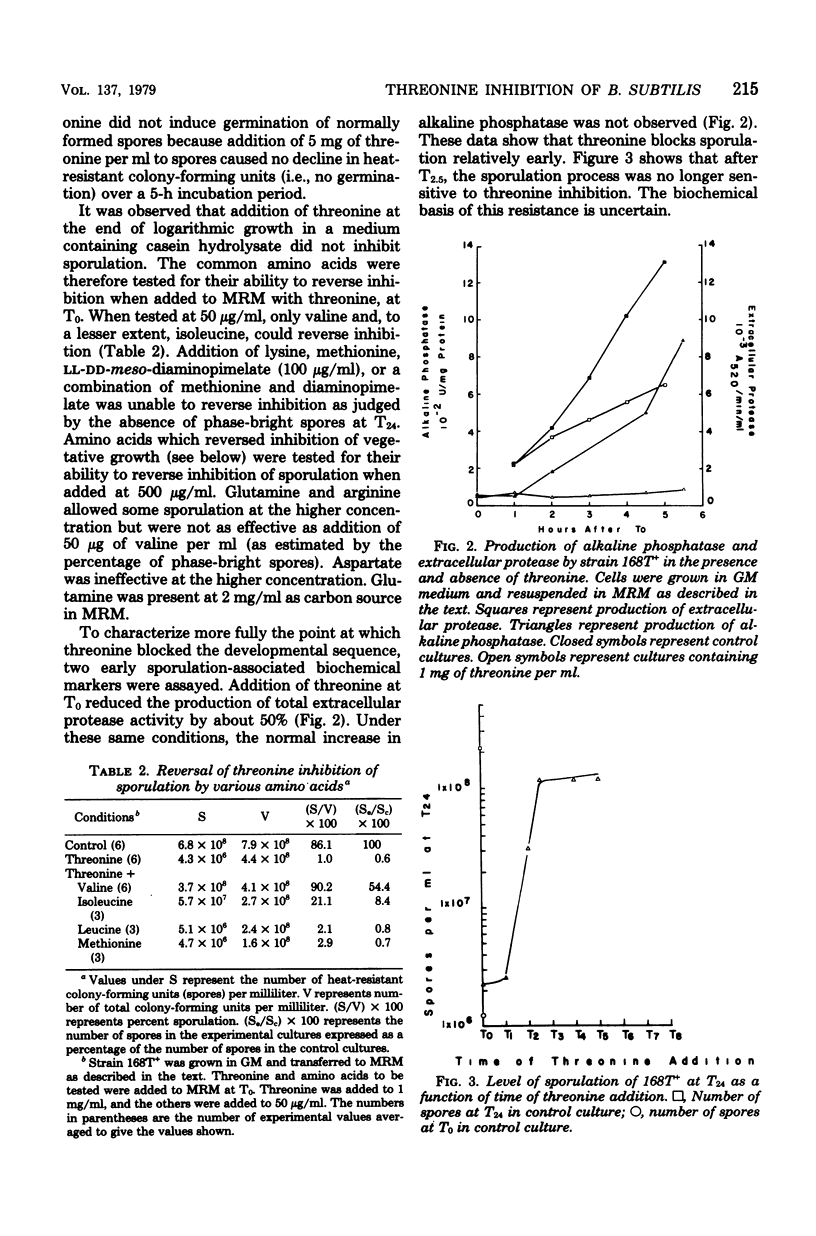
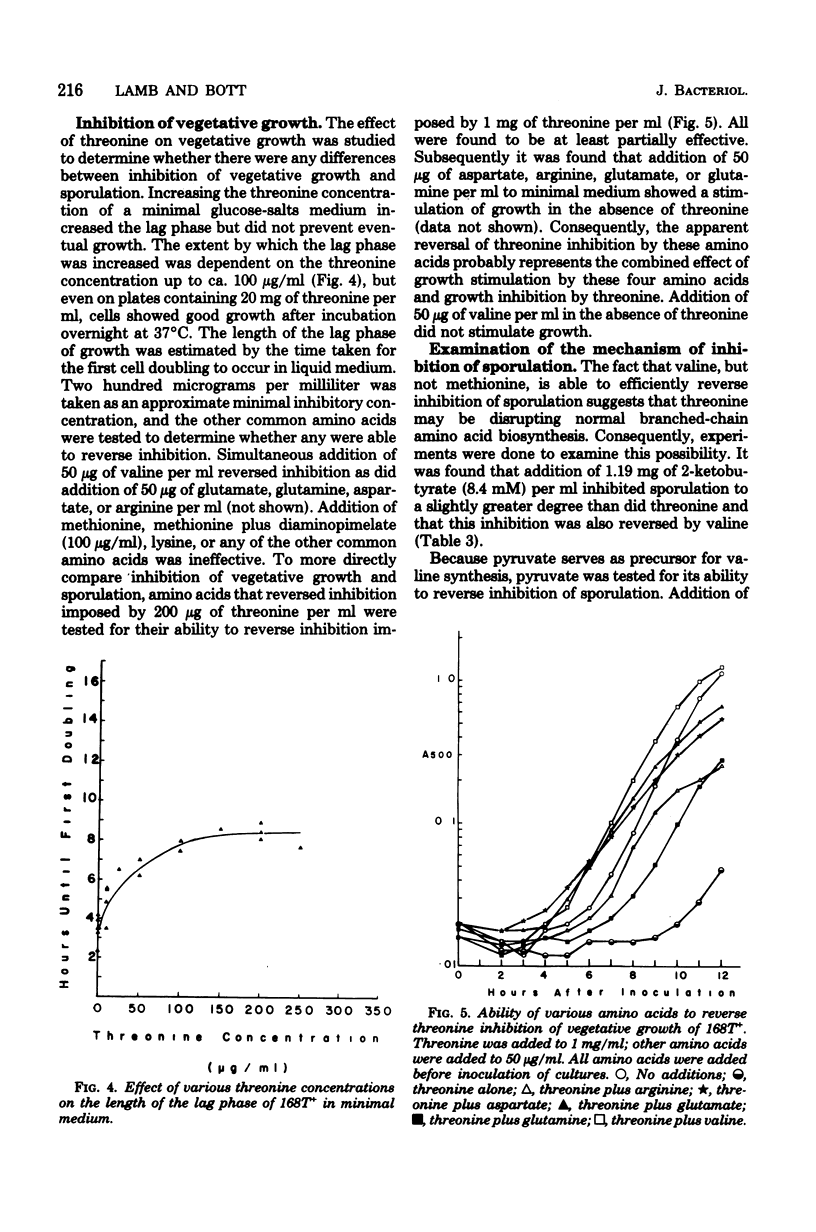
A 1-mg/ml amount of threonine (8.4 mM) inhibited growth and sporulation of Bacillus subtilis 168. Inhibition of sporulation was efficiently reversed by valine and less efficiently by pyruvate, arginine, glutamine, and isoleucine. Inhibition of vegetative growth was reversed by asparate and glutamate as well as by valine, arginine, or glutamine. Cells in minimal growth medium were inhibited only transiently by very high concentrations of threonine, whereas inhibition of sporulation was permanent. Addition of threonine prevented the normal increase in alkaline phosphatase and reduced the production of extracellular protease by about 50%, suggesting that threonine blocked the sporulation process relatively early. 2-Ketobutyrate was able to mimic the effect of threonine on sporulation. Sporulation in a strain selected for resistance to azaleucine was partially resistant. Seventy-five percent of the mutants selected for the ability to grow vegetatively in the presence of high threonine concentrations were found to be simultaneously isoleucine auxotrophs. In at least one of these mutants, the threonine resistance phenotpye could not be dissociated from the isoleucine requirement by transformation. This mutation was closely linked to a known ilvA mutation (recombination index, 0.16). This strain also had reduced intracellular threonine deaminase activity. These results suggest that threonine inhibits B. subtilis by causing valine starvation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Champney W. S., Jensen R. A. Metabolic influences on tyrosine excretion in Bacillus subtilis. J Bacteriol. 1970 Oct;104(1):351–359. doi: 10.1128/jb.104.1.351-359.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad R. S., Sokatch J. R., Jensen R. A. Relationship of metabolite inhibition of growth to flow-of-carbon patterns in nature. Life Sci. 1976 Aug 1;19(3):299–320. doi: 10.1016/0024-3205(76)90034-5. [DOI] [PubMed] [Google Scholar]
- Doering J. L., Bott K. F. Differential amino acid requirements for sporulation in Bacillus subtilis. J Bacteriol. 1972 Oct;112(1):345–355. doi: 10.1128/jb.112.1.345-355.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi R. H., Kaneko I., Igarashi R. T. Pattern of valine transfer ribonucleic acid of Bacillus subtilis under different growth conditions. J Biol Chem. 1968 Mar 10;243(5):945–951. [PubMed] [Google Scholar]
- EPHRATI-ELIZUR E., SRINIVASAN P. R., ZAMENHOF S. Genetic analysis, by means of transformation, of histidine linkage groups in Bacillus subtilis. Proc Natl Acad Sci U S A. 1961 Jan 15;47:56–63. doi: 10.1073/pnas.47.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese E., Ichikawa T., O Y. K., Freese E. B., Prasad C. Deficiencies or excesses of metabolites interfering with differentiation. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4188–4193. doi: 10.1073/pnas.71.10.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant W. D. Sporulation in Bacillus subtilis 168. Control of synthesis of alkaline phosphatase. J Gen Microbiol. 1974 Jun;82(2):363–369. doi: 10.1099/00221287-82-2-363. [DOI] [PubMed] [Google Scholar]
- Holtzclaw W. D., Chapman L. F. Properties of some norvaline-resistant mutants of Bacillus subtilis. J Gen Microbiol. 1975 Jun;88(2):289–294. doi: 10.1099/00221287-88-2-289. [DOI] [PubMed] [Google Scholar]
- Ingram L. O., Jensen R. A. Growth inhibition by L-phenylalanine in Agmenellum quadruplicatum. A clue to some amino acid interrelationships. Arch Mikrobiol. 1973 Jun 6;91(3):221–233. doi: 10.1007/BF00408909. [DOI] [PubMed] [Google Scholar]
- Jensen R. A., Calhoun D. H. Increased antimetabolite sensitivity with variation of carbon source during growth. J Bacteriol. 1978 Mar;133(3):1232–1236. doi: 10.1128/jb.133.3.1232-1236.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A., Stenmark-Cox S., Ingram L. O. Mis-regulation of 3-deoxy-D-arabino-heptulosonate 7-phosphate synthetase does not account for growth inhibition by phenylalanine in Agmenellum quadruplicatum. J Bacteriol. 1974 Dec;120(3):1124–1132. doi: 10.1128/jb.120.3.1124-1132.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRASK B. J. Methionine sulfoxide and specific inhibition of sporulation in Bacillus subtilis. J Bacteriol. 1953 Sep;66(3):374–374. doi: 10.1128/jb.66.3.374-374.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane J. F., Holmes W. M., Jensen R. A. Metabolic interlock. The dual function of a folate pathway gene as an extra-operonic gene of tryptophan biosynthesis. J Biol Chem. 1972 Mar 10;247(5):1587–1596. [PubMed] [Google Scholar]
- Kornberg A., Spudich J. A., Nelson D. L., Deutscher M. P. Origin of proteins in sporulation. Annu Rev Biochem. 1968;37:51–78. doi: 10.1146/annurev.bi.37.070168.000411. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Millet J. Characterization of a protein inhibitor of intracellular protease from Bacillus subtilis. FEBS Lett. 1977 Feb 15;74(1):59–61. doi: 10.1016/0014-5793(77)80752-7. [DOI] [PubMed] [Google Scholar]
- Ohyama K., Kaneko I., Yamakawa T., Watanabe T. Alteration in two enzymatically active forms of valyl-tRNA synthetase during the sporulation of Bacillus subtilis. J Biochem. 1977 May;81(5):1571–1574. [PubMed] [Google Scholar]
- Skarstedt M. T., Greer S. B. Threonine synthetase of Bacillus subtilis. The nature of an associated dehydratase activity. J Biol Chem. 1973 Feb 10;248(3):1032–1044. [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterlini J. M., Mandelstam J. Commitment to sporulation in Bacillus subtilis and its relationship to development of actinomycin resistance. Biochem J. 1969 Jun;113(1):29–37. doi: 10.1042/bj1130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEAS H. J. Mutants of Bacillus subtilis that require threonine or threonine plus methionine. J Bacteriol. 1950 Jan;59(1):93–104. doi: 10.1128/jb.59.1.93-104.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UMBARGER H. E., BROWN B. Threonine deamination in Escherichia coli. II. Evidence for two L-threonine deaminases. J Bacteriol. 1957 Jan;73(1):105–112. doi: 10.1128/jb.73.1.105-112.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbarger H. E. Metabolite analogs as genetic and biochemical probes. Adv Genet. 1971;16:119–140. doi: 10.1016/s0065-2660(08)60356-9. [DOI] [PubMed] [Google Scholar]
- Vapnek D., Greer S. Minor threonine dehydratase encoded within the threonine synthetic region of Bacillus subtilis. J Bacteriol. 1971 Jun;106(3):983–993. doi: 10.1128/jb.106.3.983-993.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vapnek D., Greer S. Suppression by derepression in threonine dehydratase-deficient mutants of Bacillus subtilis. J Bacteriol. 1971 May;106(2):615–625. doi: 10.1128/jb.106.2.615-625.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. B., Jr, Zahler S. A. Regulation of leucine biosynthesis in Bacillus subtilis. J Bacteriol. 1973 Nov;116(2):727–735. doi: 10.1128/jb.116.2.727-735.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. A., Bott K. F. Nutritional factors influencing the development of competence in the Bacillus subtilis transformation system. J Bacteriol. 1968 Apr;95(4):1439–1449. doi: 10.1128/jb.95.4.1439-1449.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]



