Abstract
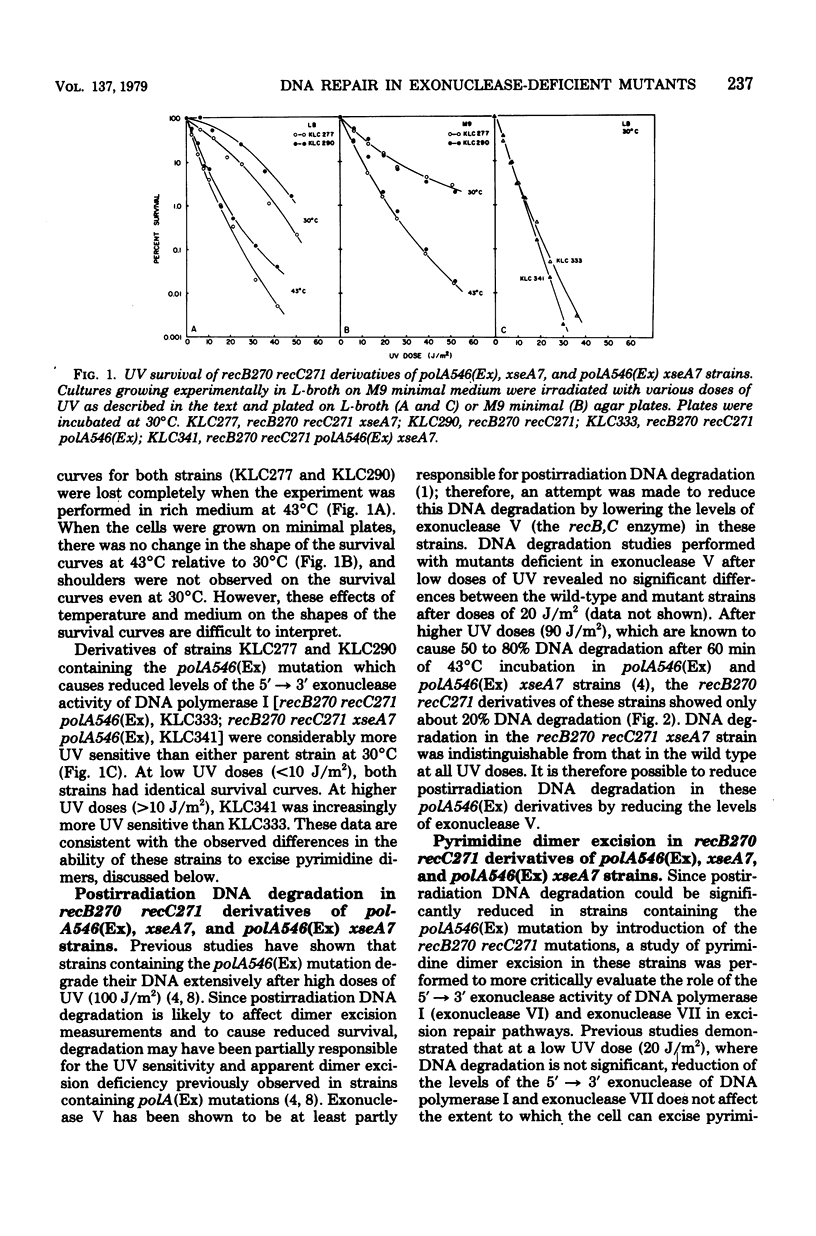
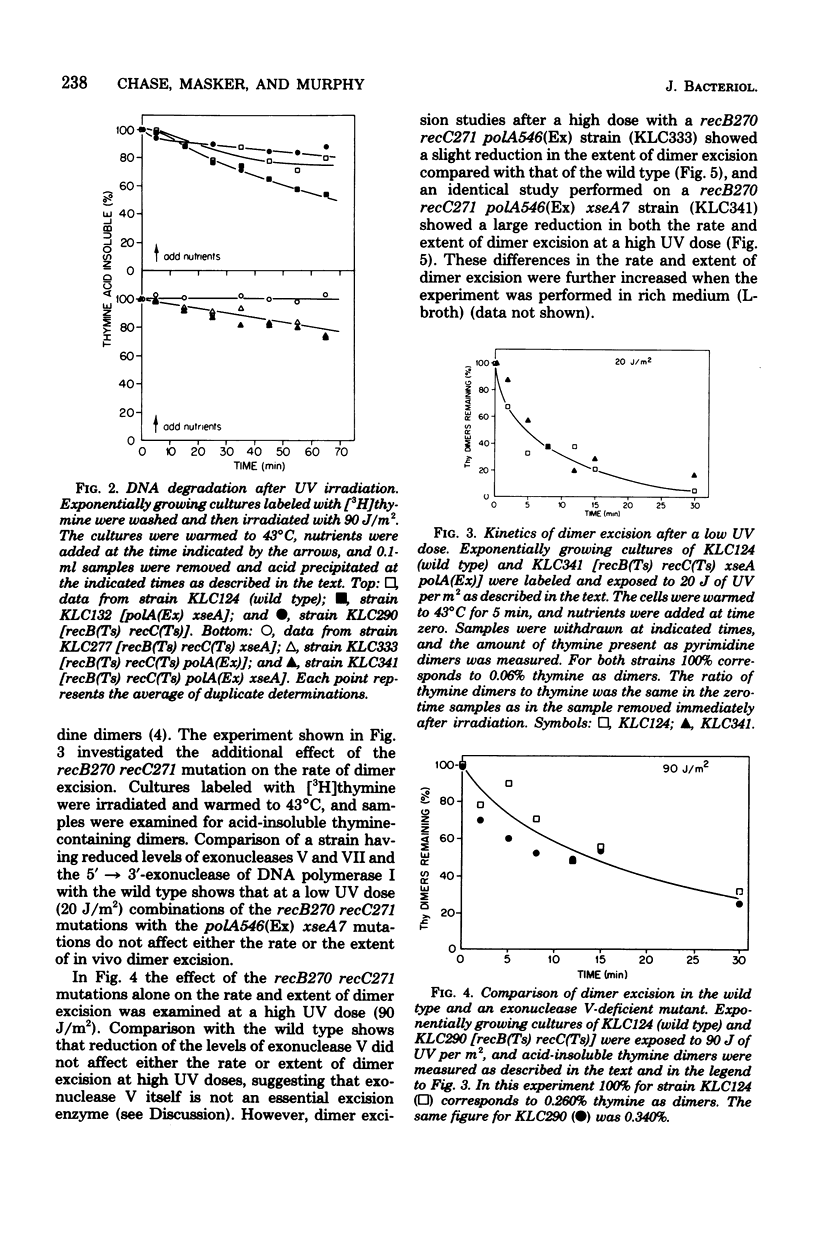
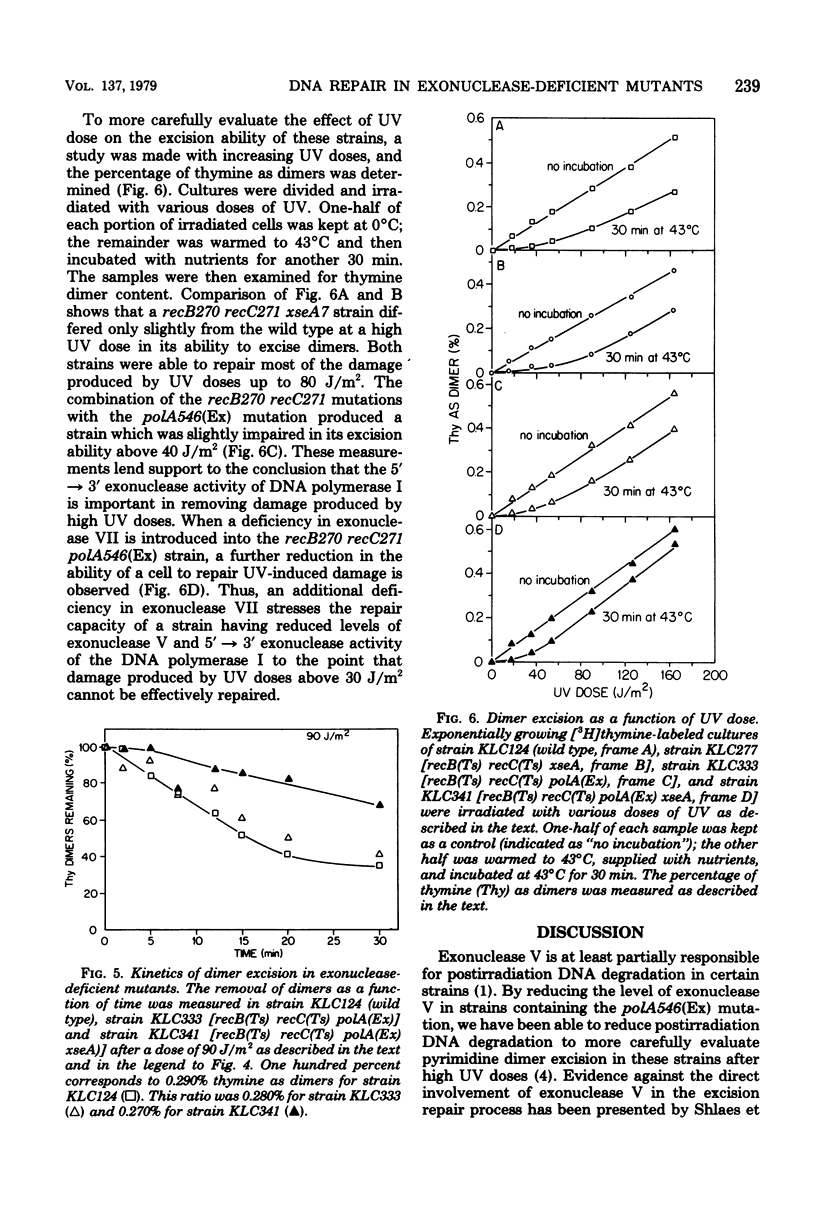
An isogenic series of Escherichia coli strains deficient in various combinations of three 5' leads to 3' exonucleases (exonuclease V, exonuclease VII, and the 5' leads to 3' exonuclease of DNA polymerase I) was constructed and examined for the ability to excise pyrimidine dimers after UV irradiation. Although the recB and recC mutations (deficient in exonuclease V) proved to be incompatible with the polA(Ex) mutation (deficient in the 5' leads to 3' exonuclease of DNA polymerase I), it was possible to reduce the level of the recB,C exonuclease by the use of temperature-sensitive recB270 recC271 mutants. It was found that, by employing strains deficient in exonuclease V, postirradiation DNA degradation could be reduced and dimer excision measurements could be facilitated. Mutants deficient in exonuclease V were found to excise dimers at a rate comparable to that of the wild type. Mutants deficient in exonuclease V and the 5' leads to 3' exonuclease of DNA polymerase I are slightly slower than the wild type at removing dimers accumulated after doses in excess of 40 J/m2. However, although strains with reduced levels of exonuclease VII excised dimers at the same rate as the wild type, the addition of an exonuclease VII deficiency to a strain with reduced levels of exonuclease V and the 5' leads to 3' exonuclease of DNA polymerase I caused a marked decrease in the rate and extent of dimer excision. These observations support previous indications that the 5' leads to 3' exonuclease of DNA polymerase I is important in dimer removal and also suggest a role for exonuclease VII in the excision repair process.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buttin G., Wright M. Enzymatic DNA degradation in E. coli: its relationship to synthetic processes at the chromosome level. Cold Spring Harb Symp Quant Biol. 1968;33:259–269. doi: 10.1101/sqb.1968.033.01.030. [DOI] [PubMed] [Google Scholar]
- Chase J. W., Masker W. E. Deoxyribonucleic acid repair in Escherichia coli mutants deficient in the 5'----3' exonuclease activity of deoxyribonucleic acid polymerase I and exonuclease VII. J Bacteriol. 1977 May;130(2):667–675. doi: 10.1128/jb.130.2.667-675.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase J. W., Richardson C. C. Escherichia coli mutants deficient in exonuclease VII. J Bacteriol. 1977 Feb;129(2):934–947. doi: 10.1128/jb.129.2.934-947.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase J. W., Richardson C. C. Exonuclease VII of Escherichia coli. Mechanism of action. J Biol Chem. 1974 Jul 25;249(14):4553–4561. [PubMed] [Google Scholar]
- Chase J. W., Richardson C. C. Exonuclease VII of Escherichia coli. Purification and properties. J Biol Chem. 1974 Jul 25;249(14):4545–4552. [PubMed] [Google Scholar]
- Cooper P. Excision-repair in mutants of Escherichia coli deficient in DNA polymerase I and/or its associated 5' leads to 3' exonuclease. Mol Gen Genet. 1977 Jan 7;150(1):1–12. doi: 10.1007/BF02425319. [DOI] [PubMed] [Google Scholar]
- Eichler D. C., Lehman I. R. On the role of ATP in phosphodiester bond hydrolysis catalyzed by the recBC deoxyribonuclease of Escherichia coli. J Biol Chem. 1977 Jan 25;252(2):499–503. [PubMed] [Google Scholar]
- Friedberg E. C., Lehman I. R. Excision of thymine dimers by proteolytic and amber fragments of E. coli DNA polymerase I. Biochem Biophys Res Commun. 1974 May 7;58(1):132–139. doi: 10.1016/0006-291x(74)90901-2. [DOI] [PubMed] [Google Scholar]
- Goldmark P. J., Linn S. Purification and properties of the recBC DNase of Escherichia coli K-12. J Biol Chem. 1972 Mar 25;247(6):1849–1860. [PubMed] [Google Scholar]
- Grossman L., Braun A., Feldberg R., Mahler I. Enzymatic repair of DNA. Annu Rev Biochem. 1975;44:19–43. doi: 10.1146/annurev.bi.44.070175.000315. [DOI] [PubMed] [Google Scholar]
- Konrad E. B., Lehman I. R. A conditional lethal mutant of Escherichia coli K12 defective in the 5' leads to 3' exonuclease associated with DNA polymerase I. Proc Natl Acad Sci U S A. 1974 May;71(5):2048–2051. doi: 10.1073/pnas.71.5.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk M., Kinross J. Conditional lethality of recA and recB derivatives of a strain of Escherichia coli K-12 with a temperature-sensitive deoxyribonucleic acid polymerase I. J Bacteriol. 1972 Mar;109(3):971–978. doi: 10.1128/jb.109.3.971-978.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Deoxyribonucleic acid polymerase: two distinct enzymes in one polypeptide. II. A proteolytic fragment containing the 5' leads to 3' exonuclease function. Restoration of intact enzyme functions from the two proteolytic fragments. J Biol Chem. 1972 Jan 10;247(1):232–240. [PubMed] [Google Scholar]
- Shlaes D. M., Anderson J. A., Barbour S. D. Excision repair properties of isogenic rec mutants of Escherichia coli K-12. J Bacteriol. 1972 Sep;111(3):723–730. doi: 10.1128/jb.111.3.723-730.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa J., Ogawa H. Structural genes of ATP-dependent deoxyribonuclease of Escherichia coli. Nat New Biol. 1972 Sep 6;239(88):14–16. doi: 10.1038/newbio239014a0. [DOI] [PubMed] [Google Scholar]
- Uyemura D., Eichler D. C., Lehman I. R. Biochemical characterization of mutant forms of DNA polymerase I from Escherichia coli. II. The polAex1 mutation. J Biol Chem. 1976 Jul 10;251(13):4085–4089. [PubMed] [Google Scholar]
- Uyemura D., Lehman I. R. Biochemical characterization of mutant forms of DNA polymerase I from Escherichia coli. I. The polA12 mutation. J Biol Chem. 1976 Jul 10;251(13):4078–4084. [PubMed] [Google Scholar]
- Willetts N. S., Mount D. W. Genetic analysis of recombination-deficient mutants of Escherichia coli K-12 carrying rec mutations cotransducible with thyA. J Bacteriol. 1969 Nov;100(2):923–934. doi: 10.1128/jb.100.2.923-934.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright M., Buttin G., Hurwitz J. The isolation and characterization from Escherichia coli of an adenosine triphosphate-dependent deoxyribonuclease directed by rec B, C genes. J Biol Chem. 1971 Nov;246(21):6543–6555. [PubMed] [Google Scholar]



