Abstract
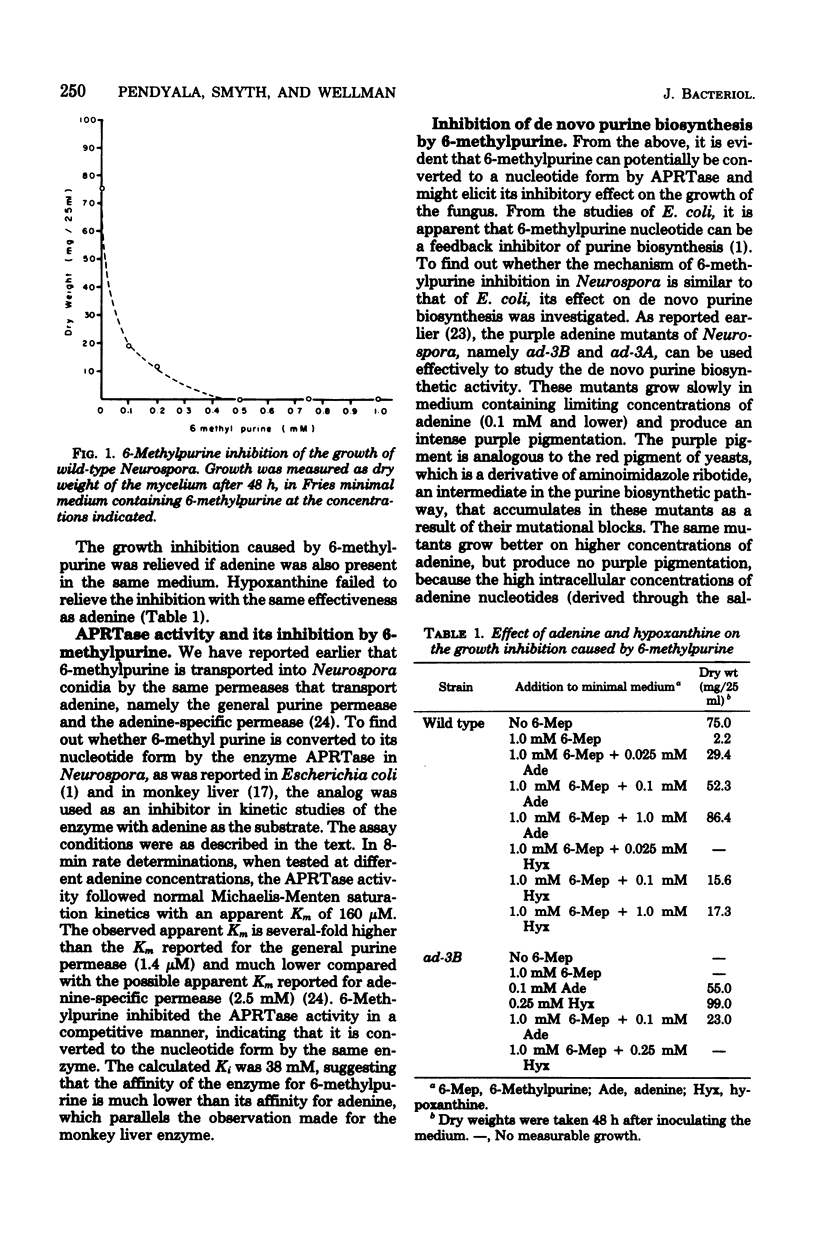
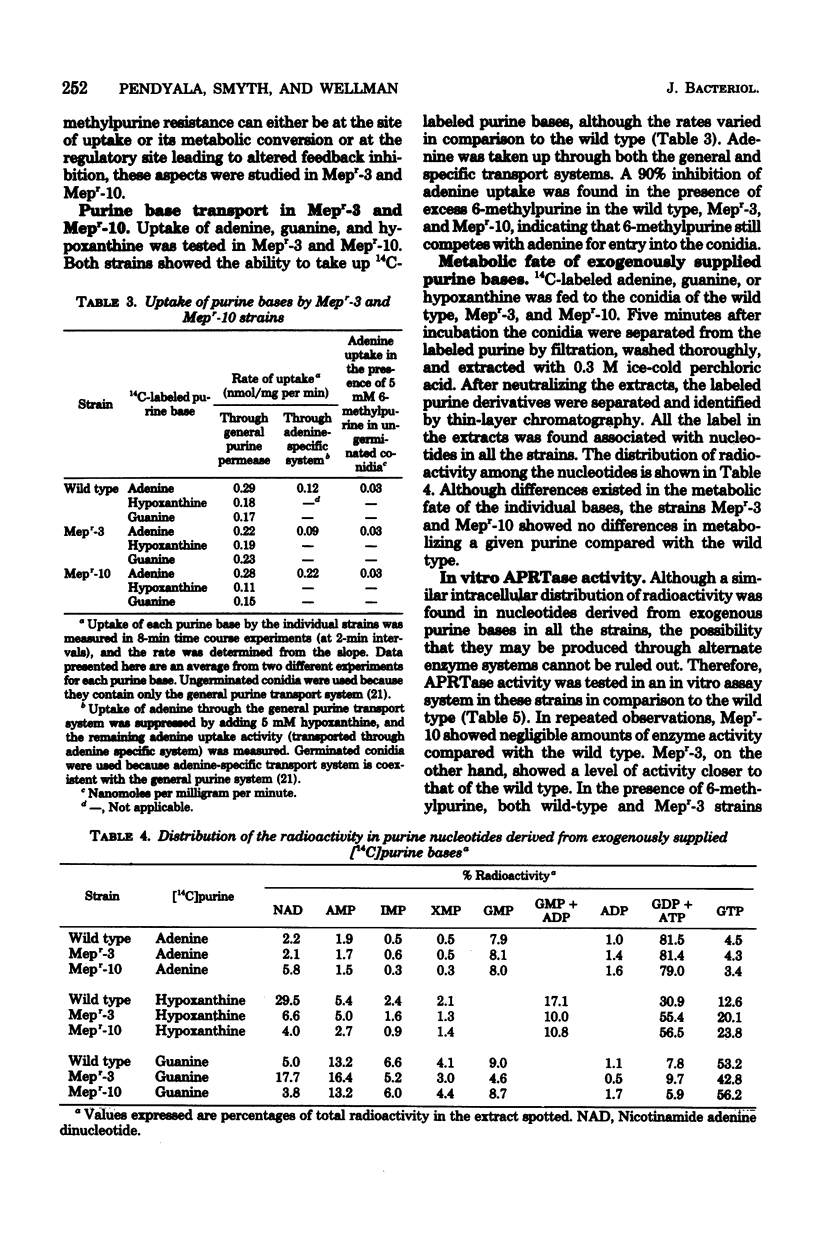
6-Methylpurine, an analog of adenine, inhibits the growth of Neurospora crassa. From kinetic studies it was found that 6-methylpurine is converted to its nucleotide form by adenine phosphoribosyltransferase (EC 2.4.2.7), and inhibits the de novo purine biosynthesis. Adenine relieves the growth inhibition caused by 6-methylpurine, whereas hypoxanthine is not very effective. Studies dealing with hypoxanthine utilization in the presence of 6-methylpurine indicated a severely reduced uptake of hypoxanthine and a general slowdown in its further metabolism. Two mutants (Mepr-3 and Mepr-10) which are resistant to 6-methylpurine were characterized. Studies of purine base uptake and the in vivo and in vitro conversion to nucleotides indicated that Mepr-10 may be an adenine phosphoribosyltransferase-defective mutant, whereas Mepr-3 may be a mutant with altered feedback response to 6-methylpurine. Both mutants showed a severely lowered hypoxanthine phosphoribosyltransferase activity, but because 6-methylpurine did not have any effect on the conversion of hypoxanthine to IMP in the wild type, it was concluded that 6-methylpurine resistance in these mutants cannot be due to lowered hypoxanthine phosphoribosyltransferase activity, but rather that the lowering of enzyme activity may be a secondary effect.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benson C. E., Love S. H., Remy C. N. Inhibition of de novo purine biosynthesis and interconversion by 6-methylpurine in Escherichia coli. J Bacteriol. 1970 Mar;101(3):872–880. doi: 10.1128/jb.101.3.872-880.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin R. D., Oliver J. M. Membrane transport of purine and pyrimidine bases and nucleosides in animal cells. Int Rev Cytol. 1975;42:287–336. doi: 10.1016/s0074-7696(08)60983-3. [DOI] [PubMed] [Google Scholar]
- Crabtree G. W., Henderson J. F. Rate-limiting steps in the interconversion of purine ribonucleotides in Ehrlich ascites tumor cells in vitro. Cancer Res. 1971 Jul;31(7):985–991. [PubMed] [Google Scholar]
- DEWEY V. C., HEINRICH M. R., MARKEES D. G., KIDDER G. W. Multiple inhibition by 6-methylpurine. Biochem Pharmacol. 1960 Jun;3:173–180. doi: 10.1016/0006-2952(60)90104-0. [DOI] [PubMed] [Google Scholar]
- Darlington A. J., Scazzocchio C. Use of analogues and the substrate-sensitivity of mutants in analysis of purine uptake and breakdown in Aspergillus nidulans. J Bacteriol. 1967 Mar;93(3):937–940. doi: 10.1128/jb.93.3.937-940.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein J., Littlefield J. W. Hypoxanthine transport in normal and hypoxanthine guanine phosphoribosyltransferase (HGPRT) deficient diploid human lymphoblasts. Exp Cell Res. 1977 May;106(2):247–251. doi: 10.1016/0014-4827(77)90169-0. [DOI] [PubMed] [Google Scholar]
- FUERST R., HSU T. C., SOMERS C. E. Studies on 4-aminopyrazolo(3, 4-d)pyrimidine: growth inhibition and relief in Neurospora crassa. J Bacteriol. 1956 Sep;72(3):387–393. doi: 10.1128/jb.72.3.387-393.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields T., Brox L. Purine and pyrimidine pool sizes and purine base utilization in human lymphocytes and cultured lymphoblasts. Can J Biochem. 1974 Jun;52(6):441–446. doi: 10.1139/o74-067. [DOI] [PubMed] [Google Scholar]
- Hochstadt-Ozer J., Stadtman E. R. The regulation of purine utilization in bacteria. II. Adenine phosphoribosyltransferase in isolated membrane preparations and its role in transport of adenine across the membrane. J Biol Chem. 1971 Sep 10;246(17):5304–5311. [PubMed] [Google Scholar]
- Hochstadt-Ozer J., Stadtman E. R. The regulation of purine utilization in bacteria. III. The involvement of purine phosphoribosyltransferases in the uptake of adenine and other nucleic acid precursors by intact resting cells. J Biol Chem. 1971 Sep 10;246(17):5312–5320. [PubMed] [Google Scholar]
- Hoffmann G. R., Malling H. V. Mutants of Neurospora crassa resistant to 8-azaguanine. J Gen Microbiol. 1974 Aug;83(2):319–326. doi: 10.1099/00221287-83-2-319. [DOI] [PubMed] [Google Scholar]
- Housset P., Nagy M. Study of the role of puring phosphoribosyltransferases in the uptake of adenine and guanine by Schizosaccharomyces pombe cells. Eur J Biochem. 1977 Feb 15;73(1):99–105. doi: 10.1111/j.1432-1033.1977.tb11295.x. [DOI] [PubMed] [Google Scholar]
- Igwegbe E. C., King V., Salary J. 6-Methylpurine-induced inhibition of sclerotia morphogenesis in Sclerotium rolfsii and its reversal by adenosine. Mycopathologia. 1977 Dec 31;62(3):153–159. doi: 10.1007/BF00444108. [DOI] [PubMed] [Google Scholar]
- Jackman L. E., Hochstadt J. Regulation of purine utilization in bacteria. VI. Characterization of hypoxanthine and guanine uptake into isolated membrane vesicles from Salmonella typhimurium. J Bacteriol. 1976 Apr;126(1):312–326. doi: 10.1128/jb.126.1.312-326.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha K. K. Genes conferring resistance to 8-aza adenine in Neurospora crassa and the variability of resistant alleles in the aza-1 locus with respect to excretion of purines. Mol Gen Genet. 1972;114(2):156–167. doi: 10.1007/BF00332786. [DOI] [PubMed] [Google Scholar]
- Krenitsky T. A., Neil S. M., Elion G. B., Hitchings G. H. Adenine phosphoribosyltransferase from monkey liver. Specificity and properties. J Biol Chem. 1969 Sep 10;244(17):4779–4784. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lomax C. A., Woods R. A. A complex genetic locus controlling purine nucleotide biosynthesis in yeast. Mol Gen Genet. 1973 Jan 24;120(2):139–149. doi: 10.1007/BF00267242. [DOI] [PubMed] [Google Scholar]
- MILLER J. H., KEMPNER E. S. EFFECTS OF AN ADENINE ANALOG ON YEAST METABOLISM. Biochim Biophys Acta. 1963 Nov 22;76:333–340. [PubMed] [Google Scholar]
- PARTRIDGE C. W., GILES N. H. Identification of major accumulation products of adenine-specific mutants of Neurospora. Arch Biochem Biophys. 1957 Mar;67(1):237–238. doi: 10.1016/0003-9861(57)90261-8. [DOI] [PubMed] [Google Scholar]
- Pendyala L., Wellman A. M. Developmental-stage-dependent adenine transport in Neurospora crassa. J Bacteriol. 1977 Aug;131(2):453–462. doi: 10.1128/jb.131.2.453-462.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendyala L., Wellman A. M. Effect of histidine on purine nucleotide synthesis and utilization in Neurospora crassa. J Bacteriol. 1975 Oct;124(1):78–85. doi: 10.1128/jb.124.1.78-85.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendyala L., Wellman A. M. Uptake and efflux of adenine and its derivatives in Neurospora crassa. Can J Microbiol. 1977 Oct;23(10):1394–1403. doi: 10.1139/m77-208. [DOI] [PubMed] [Google Scholar]
- Pickering W. R., Woods R. A. The uptake and incorporation of purines by wild-type Saccharomyces cerevisiae and a mutant resistant to 4-aminopyrazolo (3,4-d) pyrimidine. Biochim Biophys Acta. 1972 Mar 30;264(1):45–58. doi: 10.1016/0304-4165(72)90115-8. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Richey D. P. Transport of nucleosides, nucleic acid bases, choline and glucose by animal cells in culture. Biochim Biophys Acta. 1974 Dec 16;344(3-4):263–305. doi: 10.1016/0304-4157(74)90010-0. [DOI] [PubMed] [Google Scholar]
- Roy-Burman S., Visser D. W. Transport of purines and deoxyadenosine in Escherichia coli. J Biol Chem. 1975 Dec 25;250(24):9270–9275. [PubMed] [Google Scholar]
- Sabina R. L., Magill J. M., Magill C. W. Regulation of hypoxanthine transport in Neurospora crassa. J Bacteriol. 1976 Nov;128(2):598–603. doi: 10.1128/jb.128.2.598-603.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



