Abstract
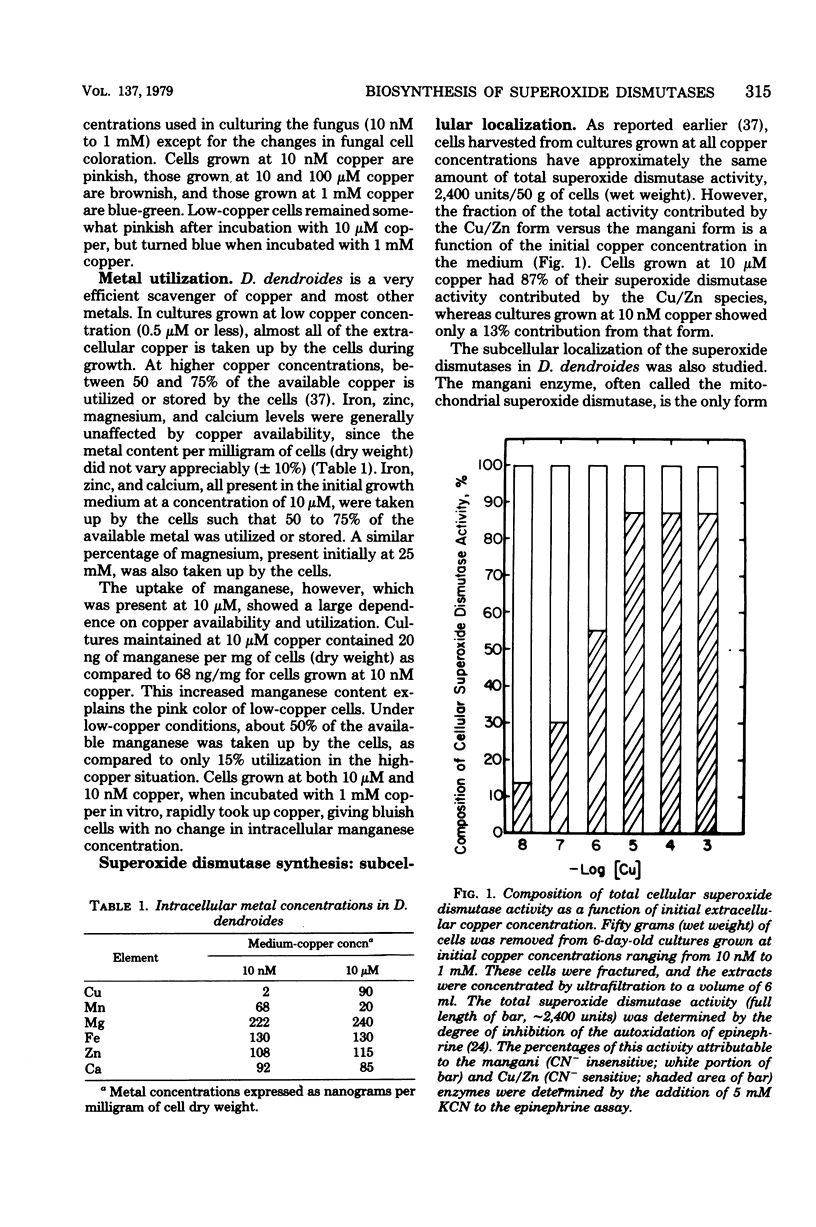
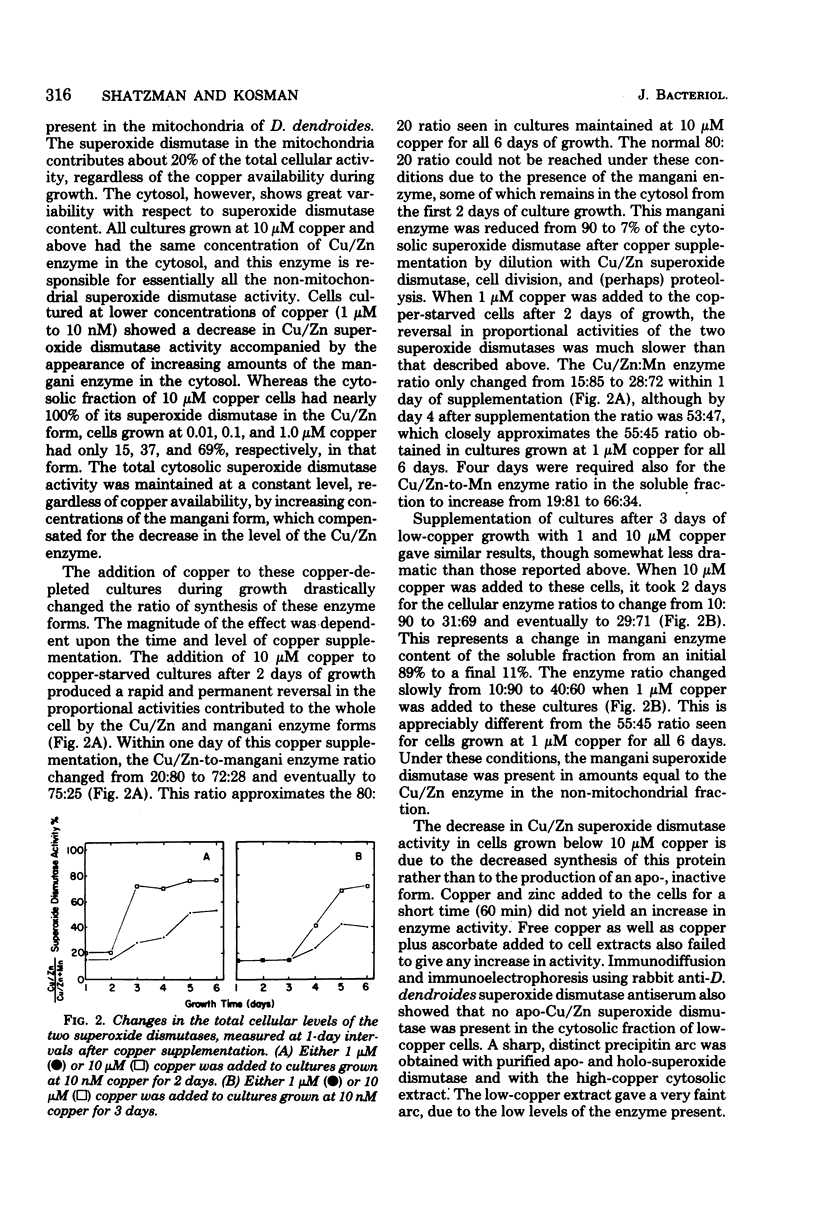
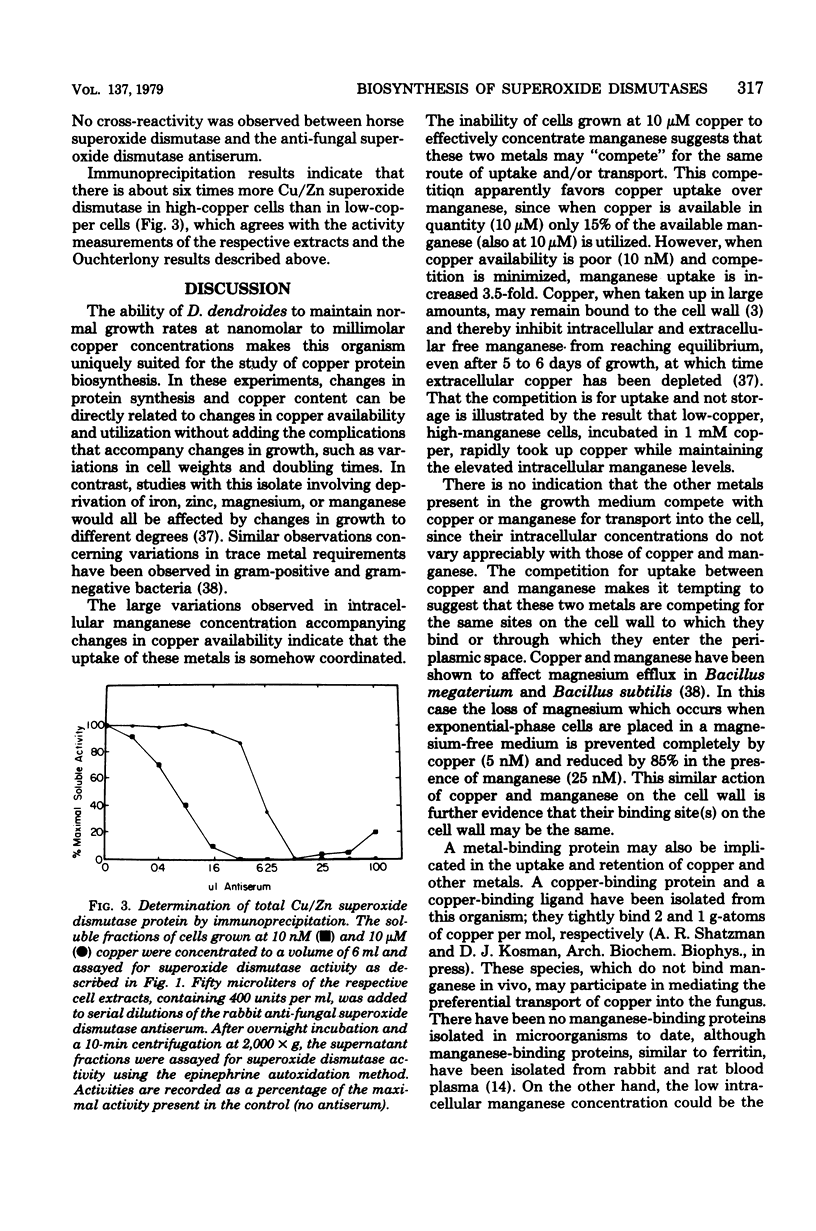
The synthesis and subcellular localization of the two superoxide dismutases of Dactylium dendroides were studied in relation to changes in copper and manganese availability. Cultures grew normally at all medium copper concentrations used (10 nM to 1 mM). In the presence of high (10 μM) copper, manganese was poorly absorbed in comparison to the other metals in the medium. However, cells grown at 10 nM copper exhibited a 3.5-fold increase in manganese content, while the concentration of the other metals remained constant. Cultures grown at 10 nM copper or more had 80% Cu/Zn enzyme and 20% mangani enzyme; the former was entirely in the cytosol, and the latter was mitochondrial. Removal of copper from the medium resulted in decreased Cu/Zn superoxide dismutase synthesis with a concomitant increase in the mangani enzyme such that total cellular superoxide dismutase activity remained constant. The mangani enzyme in excess of the 20% was present in the non-mitochondrial fraction. The mitochondria, therefore, show no variability with respect to superoxide dismutase content, whereas the soluble fraction varies from 100 to 13% Cu/Zn superoxide dismutase. Copper-starved cells that were synthesizing predominantly mangani superoxide dismutase could be switched over to mostly Cu/Zn superoxide dismutase synthesis by supplementing the medium with copper during growth. Immunoprecipitation experiments suggest that the decrease in Cu/Zn activity at low copper concentration is a result of decreased synthesis of that protein rather than the production of an inactive apoprotein.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aleman V., Handler P. Dihydroorotate dehydrogenase. I. General properties. J Biol Chem. 1967 Sep 25;242(18):4087–4096. [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Beveridge T. J., Murray R. G. Uptake and retention of metals by cell walls of Bacillus subtilis. J Bacteriol. 1976 Sep;127(3):1502–1518. doi: 10.1128/jb.127.3.1502-1518.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico R. J., Deutsch H. F. The presence of zinc in human cytocuprein and some properties of the apoprotein. J Biol Chem. 1970 Feb 25;245(4):723–727. [PubMed] [Google Scholar]
- Ehrenberg L., Fedorcsák I., Harms-Ringdahl M., Näslund M. The role of H2O2 in the reversible inhibition of RNA synthesis by thiols in E. coli. Acta Chem Scand B. 1974;28(8):960–962. doi: 10.3891/acta.chem.scand.28b-0960. [DOI] [PubMed] [Google Scholar]
- Evans GW MAJORS P. F., Cornatzer W. E. Induction of ceruloplasmin synthesis by copper. Biochem Biophys Res Commun. 1970 Dec 9;41(5):1120–1125. doi: 10.1016/0006-291x(70)90201-9. [DOI] [PubMed] [Google Scholar]
- Fee J. A., Teitelbaum H. D. Evidence that superoxide dismutase plays a role in protecting red blood cells against peroxidative hemolysis. Biochem Biophys Res Commun. 1972 Oct 6;49(1):150–158. doi: 10.1016/0006-291x(72)90022-8. [DOI] [PubMed] [Google Scholar]
- Frieden E., Osaki S., Kobayashi H. Copper proteins and oxygen. Correlations between structure and function of the copper oxidases. J Gen Physiol. 1965 Sep;49(1 Suppl):213–252. doi: 10.1085/jgp.49.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrick L. M., Dembure P. P., Garrick M. D. Interaction between the synthesis of alpha and beta globin. Eur J Biochem. 1975 Oct 15;58(2):339–350. doi: 10.1111/j.1432-1033.1975.tb02380.x. [DOI] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Induction of superoxide dismutase by molecular oxygen. J Bacteriol. 1973 May;114(2):543–548. doi: 10.1128/jb.114.2.543-548.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Goscin S. A., Fridovich I. Superoxide dismutase and oxygen toxicity in a eukaryote. J Bacteriol. 1974 Feb;117(2):456–460. doi: 10.1128/jb.117.2.456-460.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Yost F. J., Jr, Fridovich I. Superoxide dismutases of Escherichia coli: intracellular localization and functions. J Bacteriol. 1973 Sep;115(3):987–991. doi: 10.1128/jb.115.3.987-991.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. G., Evans D. J., Fritze K. Manganese proteins in blood plasma. Biochim Biophys Acta. 1973 Sep 14;320(2):486–493. doi: 10.1016/0304-4165(73)90329-2. [DOI] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Enzymatic defenses against the toxicity of oxygen and of streptonigrin in Escherichia coli. J Bacteriol. 1977 Mar;129(3):1574–1583. doi: 10.1128/jb.129.3.1574-1583.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- Keele B. B., Jr, McCord J. M., Fridovich I. Superoxide dismutase from escherichia coli B. A new manganese-containing enzyme. J Biol Chem. 1970 Nov 25;245(22):6176–6181. [PubMed] [Google Scholar]
- MOHAMED M. S., GREENBERG D. M. Isolation of purified copper protein from horse liver. J Gen Physiol. 1954 Mar;37(4):433–439. doi: 10.1085/jgp.37.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus Z., Miller G., Avigad G. Effect of culture conditions on the production of D-galactose oxidase by Dactylium dendroides. Appl Microbiol. 1965 Sep;13(5):686–693. doi: 10.1128/am.13.5.686-693.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- McCord J. M. Iron- and manganese-containing superoxide dismutases: structure, distribution, and evolutionary relationships. Adv Exp Med Biol. 1976;74:540–550. doi: 10.1007/978-1-4684-3270-1_45. [DOI] [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. The purification and properties of superoxide dismutase from Neurospora crassa. J Biol Chem. 1972 Jun 10;247(11):3410–3414. [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972 May 25;247(10):3170–3175. [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- PENNINGTON R. J. Biochemistry of dystrophic muscle. Mitochondrial succinate-tetrazolium reductase and adenosine triphosphatase. Biochem J. 1961 Sep;80:649–654. doi: 10.1042/bj0800649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTER H., AINSWORTH S. Reaction of brain copper proteins with sodium diethyldithiocarbamate in normal and in hepatolenticular degeneration. Proc Soc Exp Biol Med. 1958 Jun;98(2):277–280. doi: 10.3181/00379727-98-24015. [DOI] [PubMed] [Google Scholar]
- PORTER H., AINSWORTH S. The isolation of the coppercontaining protein cerebrocuprein I from normal human brain. J Neurochem. 1959 Dec;5:91–98. doi: 10.1111/j.1471-4159.1959.tb13337.x. [DOI] [PubMed] [Google Scholar]
- PORTER H., FOLCH J. Cerebrocuprein I. A copper-containing protein isolated from brain. J Neurochem. 1957;1(3):260–271. doi: 10.1111/j.1471-4159.1957.tb12081.x. [DOI] [PubMed] [Google Scholar]
- PORTER H., SWEENEY M., PORTER E. M. HUMAN HEPATOCUPREIN. ISOLATION OF A COPPER PROTEIN FROM THE SUBCELLULAR SOLUBLE FRACTION OF ADULT HUMAN LIVER. Arch Biochem Biophys. 1964 May;105:319–325. doi: 10.1016/0003-9861(64)90014-1. [DOI] [PubMed] [Google Scholar]
- Peeters-Joris C., Vandevoorde A. M., Baudhuin P. Subcellular localization of superoxide dismutase in rat liver. Biochem J. 1975 Jul;150(1):31–39. doi: 10.1042/bj1500031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAJAGOPALAN K. V., FRIDOVICH I., HANDLER P. Hepatic aldehyde oxidase. I. Purification and properties. J Biol Chem. 1962 Mar;237:922–928. [PubMed] [Google Scholar]
- Rest R. F., Spitznagel J. K. Subcellular distribution of superoxide dismutases in human neutrophils. Influence of myeloperoxidase on the measurement of superoxide dismutase activity. Biochem J. 1977 Aug 15;166(2):145–153. doi: 10.1042/bj1660145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- Shatzman A. R., Kosman D. J. Regulation of galactose oxidase synthesis and secretion in Dactylium dendroides: effects of pH and culture density. J Bacteriol. 1977 Apr;130(1):455–463. doi: 10.1128/jb.130.1.455-463.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatzman A. R., Kosman D. J. The utilization of copper and its role in the biosynthesis of copper-containing proteins in the fungus, Dactylium dendroides. Biochim Biophys Acta. 1978 Nov 15;544(1):163–179. doi: 10.1016/0304-4165(78)90220-9. [DOI] [PubMed] [Google Scholar]
- Webb M. The influence of certain trace metals on bacterial growth and magnesium utilization. J Gen Microbiol. 1968 May;51(3):325–335. doi: 10.1099/00221287-51-3-325. [DOI] [PubMed] [Google Scholar]
- Weisiger R. A., Fridovich I. Superoxide dismutase. Organelle specificity. J Biol Chem. 1973 May 25;248(10):3582–3592. [PubMed] [Google Scholar]
- Zähringer J., Baliga B. S., Munro H. N. Novel mechanism for translational control in regulation of ferritin synthesis by iron. Proc Natl Acad Sci U S A. 1976 Mar;73(3):857–861. doi: 10.1073/pnas.73.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]


