Abstract
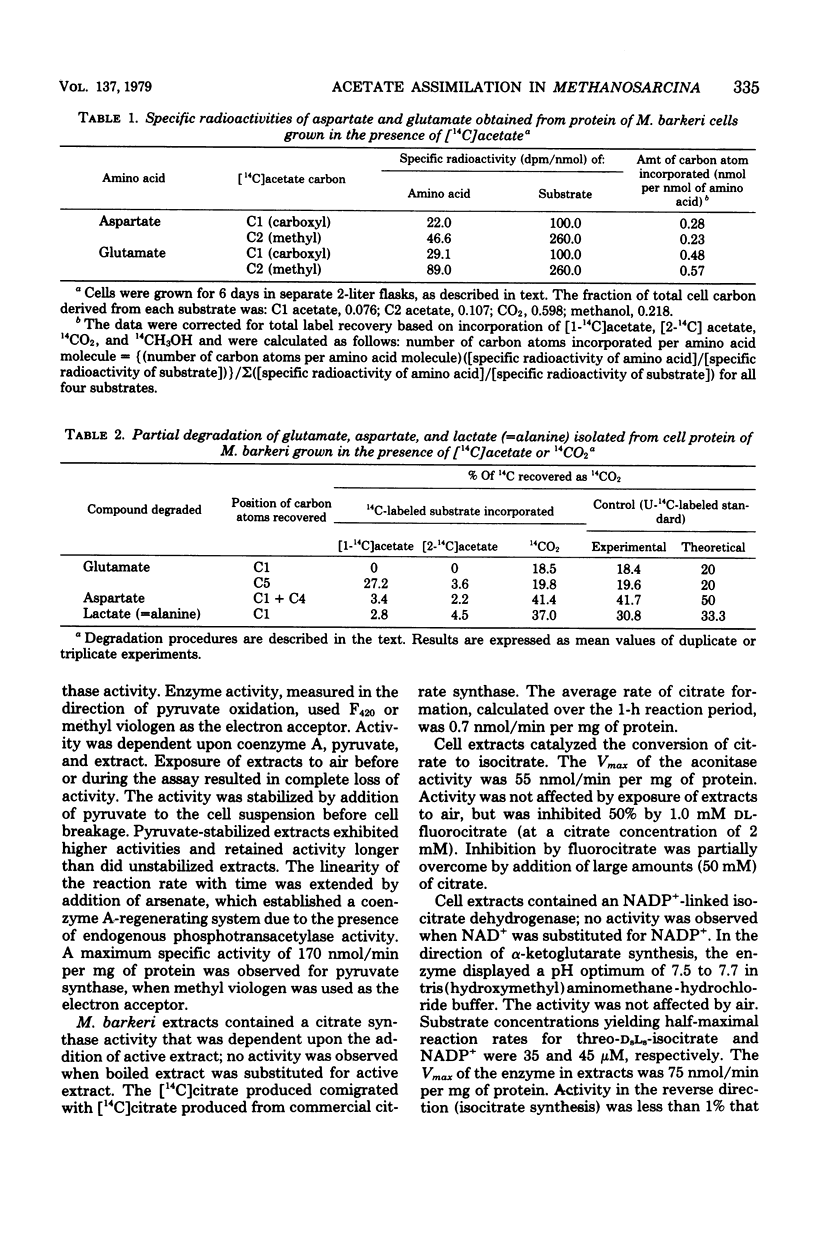
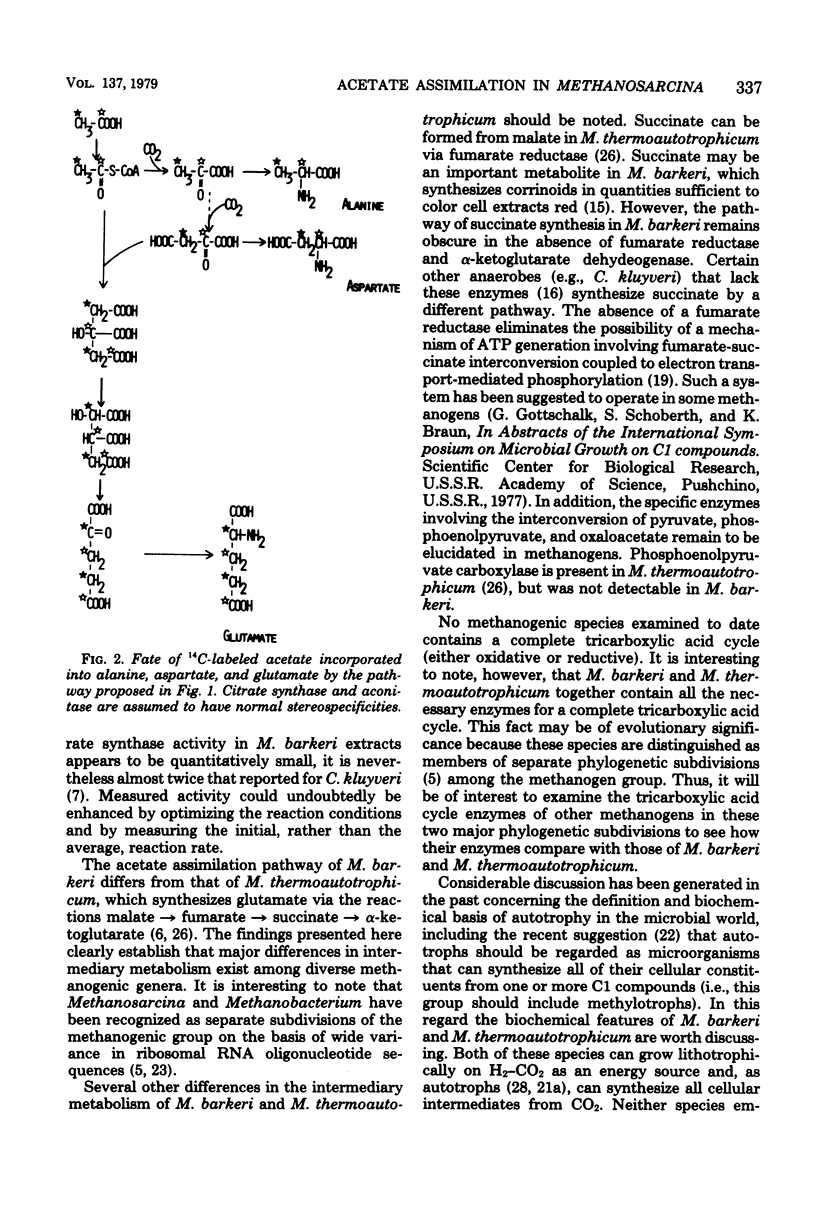
The pathway of acetate assimilation in Methanosarcina barkeri was determined from analysis of the position of label in alanine, aspartate, and glutamate formed in cells grown in the presence of [14C]acetate and by measurement of enzyme activities in cell extracts. The specific radioactivity of glutamate from cells grown on [1-14C]- or [2-14C]acetate was approximately twice that of aspartate. The methyl and carboxyl carbons of acetate were incorporated into aspartate and glutamate to similar extents. Degradation studies revealed that acetate was not significantly incorporated into the C1 of alanine, C1 or C4 of aspartate, or C1 of glutamate. The C5 of glutamate, however, was partially derived from the carboxyl carbon of acetate. Cell extracts were found to contain the following enzyme activities, in nanomoles per minute per milligram of protein at 37 degrees C: F420-linked pyruvate synthase, 170; citrate synthase, 0.7; aconitase, 55; oxidized nicotinamide adenine dinucleotide phosphate-linked isocitrate dehydrogenase, 75; and oxidized nicotinamide adenine dinucleotide-linked malate dehydrogenase, 76. The results indicate that M. barkeri assimilates acetate into alanine and aspartate via pyruvate and oxaloacetate and into glutamate via citrate, isocitrate, and alpha-ketoglutarate. The data reveal differences in the metabolism of M. barkeri and Methanobacterium thermoautotrophicum and similarities in the assimilation of acetate between M. barkeri and other anaerobic bacteria, such as Clostridium kluyveri.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beuscher N., Mayer F., Gottschalk G. Citrate lyase from Rhodopseudomonas gelatinosa: purification, electron microscopy and subunit structure. Arch Microbiol. 1974;100(4):307–328. doi: 10.1007/BF00446325. [DOI] [PubMed] [Google Scholar]
- Bishop R., Sims A. P. Problems in the determination of the specific radioactivity of uniformly 14-C-labelled amino-acids after their reaction with ninhydrin. Analyst. 1975 Jun;100(1191):369–376. doi: 10.1039/an9750000369. [DOI] [PubMed] [Google Scholar]
- Daniels L., Fuchs G., Thauer R. K., Zeikus J. G. Carbon monoxide oxidation by methanogenic bacteria. J Bacteriol. 1977 Oct;132(1):118–126. doi: 10.1128/jb.132.1.118-126.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels L., Zeikus J. G. One-carbon metabolism in methanogenic bacteria: analysis of short-term fixation products of 14CO2 and 14CH3OH incorporated into whole cells. J Bacteriol. 1978 Oct;136(1):75–84. doi: 10.1128/jb.136.1.75-84.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox G. E., Magrum L. J., Balch W. E., Wolfe R. S., Woese C. R. Classification of methanogenic bacteria by 16S ribosomal RNA characterization. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4537–4541. doi: 10.1073/pnas.74.10.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs G., Stupperich E., Thauer R. K. Acetate assimilation and the synthesis of alanine, aspartate and glutamate in Methanobacterium thermoautotrophicum. Arch Microbiol. 1978 Apr 27;117(1):61–66. doi: 10.1007/BF00689352. [DOI] [PubMed] [Google Scholar]
- Gottschalk G., Barker H. A. Synthesis of glutamate and citrate by Clostridium kluyveri. A new type of citrate synthase. Biochemistry. 1966 Apr;5(4):1125–1133. doi: 10.1021/bi00868a003. [DOI] [PubMed] [Google Scholar]
- HANSON K. R., ROSE I. A. THE ABSOLUTE STEREOCHEMICAL COURSE OF CITRIC ACID BIOSYNTHESIS. Proc Natl Acad Sci U S A. 1963 Nov;50:981–988. doi: 10.1073/pnas.50.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOARE D. S. The photo-assimilation of acetate by Rhodospirillum rubrum. Biochem J. 1963 May;87:284–301. doi: 10.1042/bj0870284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEMBLE A. R., MACPHERSON H. T. Determination of monoamino monocarboxylic acids by quantitative paper chromatography. Biochem J. 1954 Apr;56(4):548–555. doi: 10.1042/bj0560548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Smith A. J., London J., Stanier R. Y. Biochemical basis of obligate autotrophy in blue-green algae and thiobacilli. J Bacteriol. 1967 Oct;94(4):972–983. doi: 10.1128/jb.94.4.972-983.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern J. R., Bambers G. Glutamate biosynthesis in anaerobic bacteria. I. The citrate pathways of glutamate synthesis in Clostridium kluyveri. Biochemistry. 1966 Apr;5(4):1113–1118. doi: 10.1021/bi00868a001. [DOI] [PubMed] [Google Scholar]
- Stern J. R., Hegre C. S., Bambers G. Glutamate biosynthesis in anaerobic bacteria. II. Stereospecificity of aconitase and citrate synthetase of Clostridium kluyveri. Biochemistry. 1966 Apr;5(4):1119–1124. doi: 10.1021/bi00868a002. [DOI] [PubMed] [Google Scholar]
- TOMLINSON N. Carbon dioxide and acetate utilization by Clostridium kluyveri. III. A new path of glutamic acid synthesis. J Biol Chem. 1954 Aug;209(2):605–609. [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WACHSMAN J. T., BARKER H. A. Tracer experiments on glutamate fermentation by Clostridium tetanomorphum. J Biol Chem. 1955 Dec;217(2):695–702. [PubMed] [Google Scholar]
- Weimer P. J., Zeikus J. G. Acetate metabolism in Methanosarcina barkeri. Arch Microbiol. 1978 Nov 13;119(2):175–182. doi: 10.1007/BF00964270. [DOI] [PubMed] [Google Scholar]
- Weimer P. J., Zeikus J. G. One carbon metabolism in methanogenic bacteria. Cellular characterization and growth of Methanosarcina barkeri. Arch Microbiol. 1978 Oct 4;119(1):49–57. doi: 10.1007/BF00407927. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Fox G. E. Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5088–5090. doi: 10.1073/pnas.74.11.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe R. S. Microbial formation of methane. Adv Microb Physiol. 1971;6:107–146. doi: 10.1016/s0065-2911(08)60068-5. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G., Fuchs G., Kenealy W., Thauer R. K. Oxidoreductases involved in cell carbon synthesis of Methanobacterium thermoautotrophicum. J Bacteriol. 1977 Nov;132(2):604–613. doi: 10.1128/jb.132.2.604-613.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G. The biology of methanogenic bacteria. Bacteriol Rev. 1977 Jun;41(2):514–541. doi: 10.1128/br.41.2.514-541.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Wolfe R. S. Methanobacterium thermoautotrophicus sp. n., an anaerobic, autotrophic, extreme thermophile. J Bacteriol. 1972 Feb;109(2):707–715. doi: 10.1128/jb.109.2.707-713.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



