Abstract
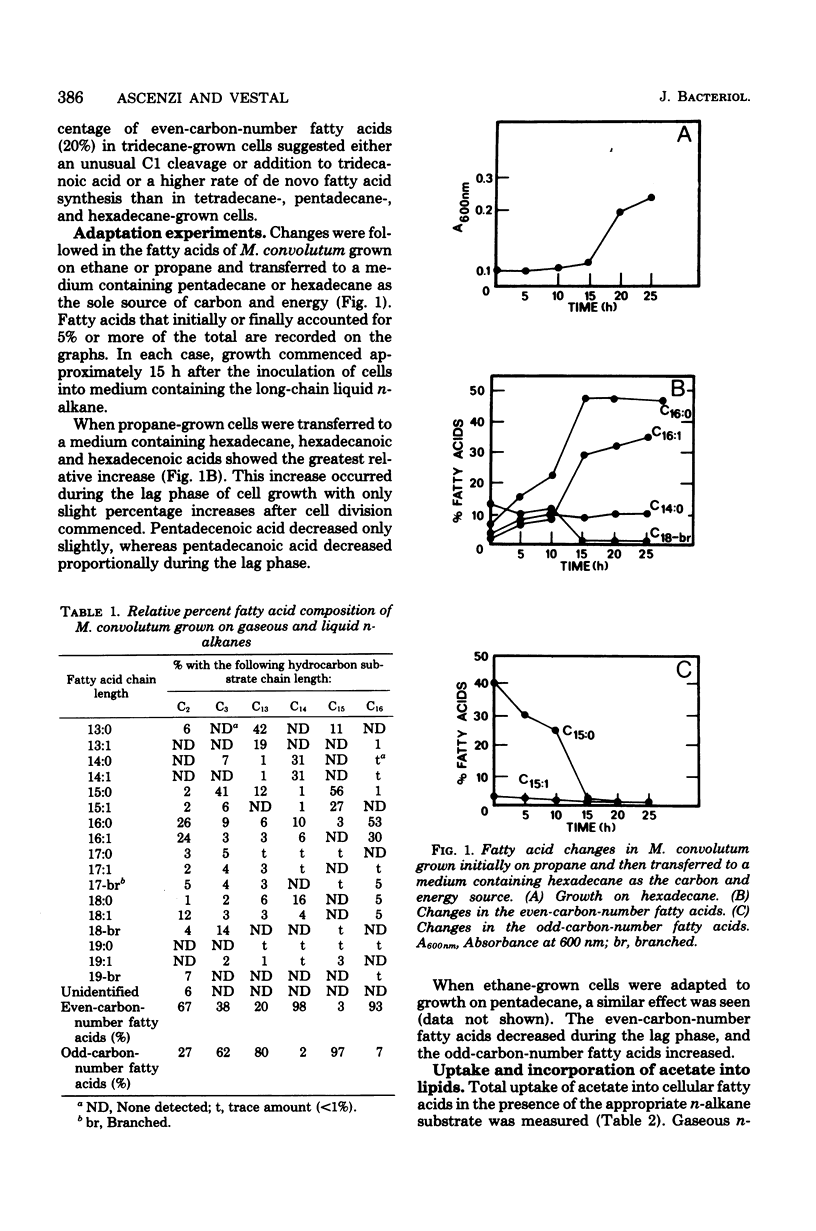
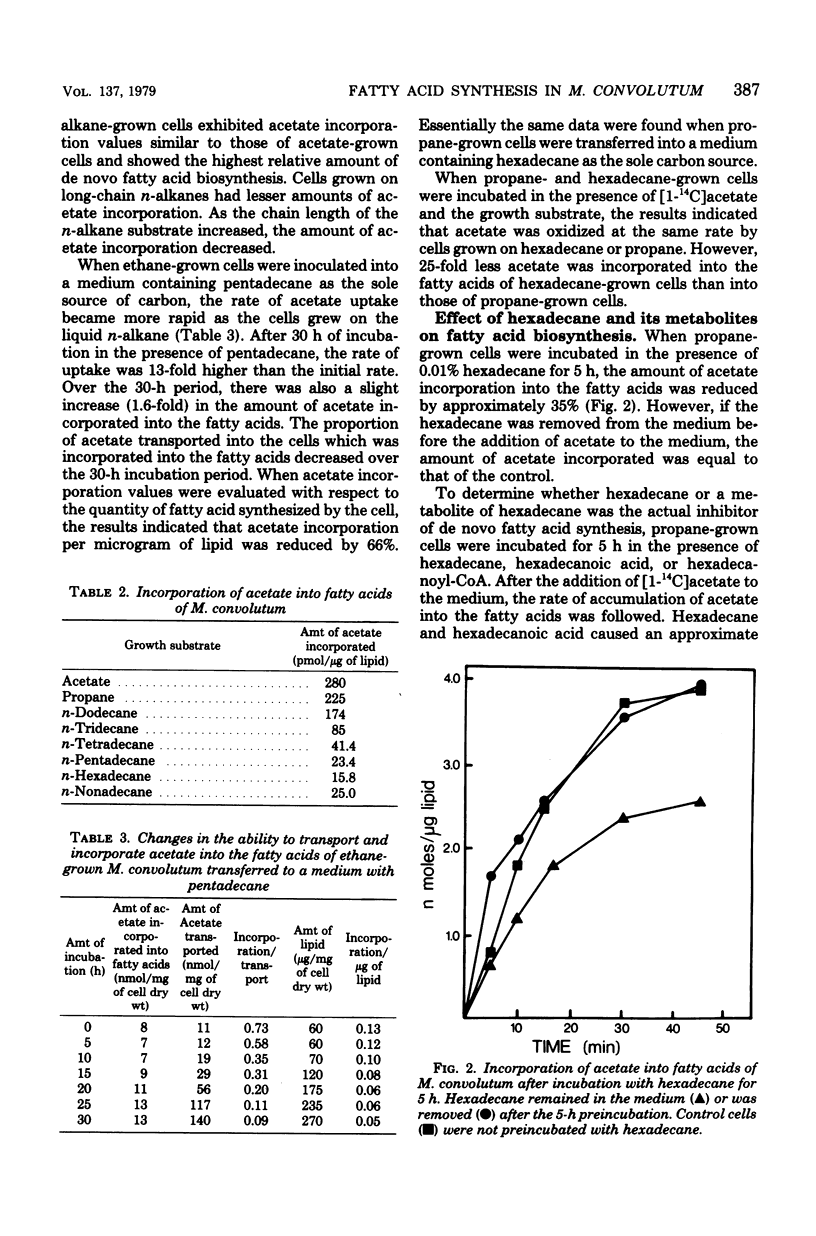
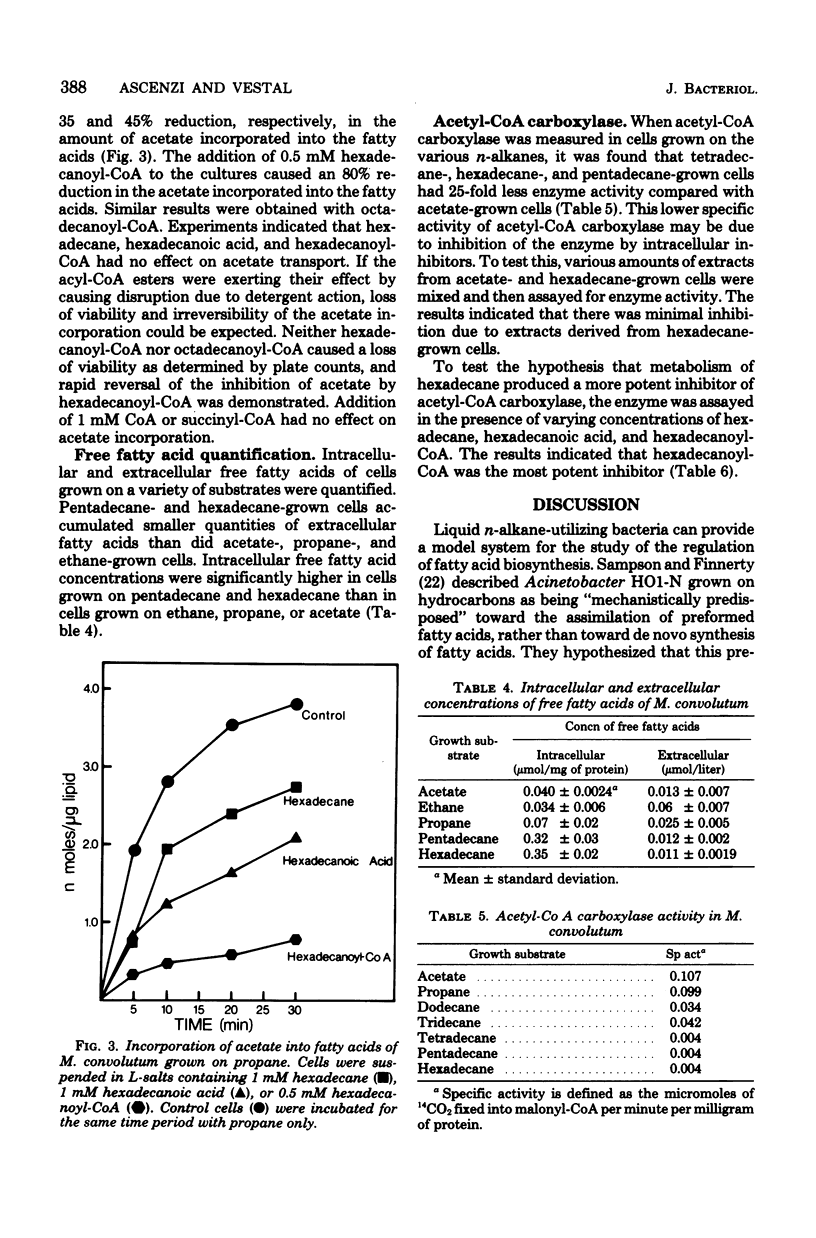
When Mycobacterium convolutum R22 was grown on the n-alkanes C13 through C16, the predominant fatty acids were of the same chain length as the growth substrate. Cells grown on C13 through C16 n-alkanes incorporated between 15 and 85 pmol of acetate per microgram of lipid into the fatty acids, whereas acetate- or propane-grown cells incorporated 280 and 255 pmol of acetate per microgram of lipid, respectively. In vivo experiments demonstrated that hexadecane, hexadecanoic acid, and hexadecanoylcoenzyme A (CoA) all inhibited de novo fatty acid synthesis. Hexadecanoyl-CoA was the most potent inhibitor. Hexadecane and hexadecanoic acid inhibited acetyl-CoA carboxylase by up to 37 and 39%, respectively, at 1 mM. Hexadecanoyl-CoA inhibited the enzyme activity by 65% at 50 micrometer. Cells that were grown on C14 through C16 n-alkanes had about 25 times less acetyl-CoA carboxylase activity than did cells grown on acetate or propane, suggesting repressed levels of the enzyme. Hexadecane- or pentadecane-grown cells were found to have 5 to 10 times more intracellular free fatty acid than cells grown on acetate, propane, or ethane.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Birnbaum J. Coenzyme repression of acetyl-CoA carboxylase by (+)-biotin. Arch Biochem Biophys. 1969 Jul;132(2):436–441. doi: 10.1016/0003-9861(69)90386-5. [DOI] [PubMed] [Google Scholar]
- Birnbaum J. Repression of acetyl-coenzyme A carboxylase by unsaturated fatty acids: relationship to coenzyme repression. J Bacteriol. 1970 Oct;104(1):171–176. doi: 10.1128/jb.104.1.171-176.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney J. J., Proby C. M. Fatty acid composition of Cladosporium resinae grown on glucose and on hydrocarbons. J Bacteriol. 1971 Nov;108(2):777–781. doi: 10.1128/jb.108.2.777-781.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOLE V. P. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J Clin Invest. 1956 Feb;35(2):150–154. doi: 10.1172/JCI103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K. R., Perry J. J. Effect of Substrate on the Fatty Acid Composition of Hydrocarbon- and Ketone-utilizing Microorganisms. J Bacteriol. 1968 Aug;96(2):318–321. doi: 10.1128/jb.96.2.318-321.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K. R., Perry J. J. Effect of substrate on the fatty acid composition of hydrocabon-utilizing microorganisms. J Bacteriol. 1967 Dec;94(6):1919–1923. doi: 10.1128/jb.94.6.1919-1923.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill C. O., Ratledge C. Regulation of de novo fatty acid biosynthesis in the n-alkane-utilizing yeast, Candida 107. J Gen Microbiol. 1973 Oct;78(2):337–347. doi: 10.1099/00221287-78-2-337. [DOI] [PubMed] [Google Scholar]
- Hallas L. E., Vestal J. R. The growth of Mycobacterium convolutum on solid n-alkane substrates: effect on cellular lipid composition. Can J Microbiol. 1978 Oct;24(10):1197–1203. doi: 10.1139/m78-194. [DOI] [PubMed] [Google Scholar]
- JAMES A. T. Qualitative and quantitative determination of the fatty acids by gas-liquid chromatography. Methods Biochem Anal. 1960;8:1–59. doi: 10.1002/9780470110249.ch1. [DOI] [PubMed] [Google Scholar]
- King D. H., Perry J. J. The origin of fatty acids in the hydrocarbon-utilizing microorganism Mycobacterium vaccae. Can J Microbiol. 1975 Jan;21(1):85–89. doi: 10.1139/m75-012. [DOI] [PubMed] [Google Scholar]
- LEADBETTER E. R., FOSTER J. W. Studies on some methane-utilizing bacteria. Arch Mikrobiol. 1958;30(1):91–118. doi: 10.1007/BF00509229. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Makula R. A., Finnerty W. R. Microbial assimilation of hydrocarbons: cellular distribution of fatty acids. J Bacteriol. 1972 Oct;112(1):398–407. doi: 10.1128/jb.112.1.398-407.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makula R., Finnerty W. R. Microbial assimilation of hydrocarbons. I. Fatty acids derived from normal alkanes. J Bacteriol. 1968 Jun;95(6):2102–2107. doi: 10.1128/jb.95.6.2102-2107.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makula R., Finnerty W. R. Microbial assimilation of hydrocarbons. II. Fatty acids derived from 1-alkenes. J Bacteriol. 1968 Jun;95(6):2108–2111. doi: 10.1128/jb.95.6.2108-2111.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. L., Levy H. R. Rat mammary acetyl coenzyme A carboxylase. I. Isolation and characterization. J Biol Chem. 1969 May 10;244(9):2334–2342. [PubMed] [Google Scholar]
- NOVAK M. COLORIMETRIC ULTRAMICRO METHOD FOR THE DETERMINATION OF FREE FATTY ACIDS. J Lipid Res. 1965 Jul;6:431–433. [PubMed] [Google Scholar]
- Patrick M. A., Dugan P. R. Influence of hydrocarbons and derivatives on the polar lipid fatty acids of an Acinetobacter isolate. J Bacteriol. 1974 Jul;119(1):76–81. doi: 10.1128/jb.119.1.76-81.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K., Sato K. A simple colorimetric estimation of lipids with sodium dichromate. J Biochem. 1966 Jun;59(6):619–621. doi: 10.1093/oxfordjournals.jbchem.a128351. [DOI] [PubMed] [Google Scholar]
- Sampson K. L., Finnerty W. R. Regulation of fatty acid biosynthesis in the hydrocarbon oxidizing microorganism, Acinetobacter sp. Arch Microbiol. 1974;99(3):203–220. doi: 10.1007/BF00696235. [DOI] [PubMed] [Google Scholar]
- Vestal J. R., Perry J. J. Divergent metabolic pathways for propane and propionate utilization by a soil isolate. J Bacteriol. 1969 Jul;99(1):216–221. doi: 10.1128/jb.99.1.216-221.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestal J. R., Perry J. J. Effect of substrate on the lipids of the hydrocarbon-utilizing Mycobacterium vaccae. Can J Microbiol. 1971 Apr;17(4):445–449. doi: 10.1139/m71-075. [DOI] [PubMed] [Google Scholar]
- Weeks G., Wakil S. J. Studies on the control of fatty acid metabolism. II. The inhibition of fatty acid synthesis in Lactobacillus plantarum by exogenous fatty acid. J Biol Chem. 1970 Apr 25;245(8):1913–1921. [PubMed] [Google Scholar]



