Abstract
Members of the major histocompatibility complex (MHC) class I family of proteins are well known for their central role in the adaptive immune system, where they present self and non-self peptides for immune surveillance. Although the brain has been long considered immune privileged, in part because of an apparent lack of neuronal MHC class I, it has since been shown that MHC class I proteins are expressed by normal, uninfected neurons. Moreover, expression of MHC class I is unusually dynamic in the developing and adult brain, and MHC class I levels in neurons can be regulated by endogenous and exogenous electrical activity. Unexpectedly, several recent studies find that MHC class I is required for distinct activity-dependent events during brain development, adult plasticity, and in response to injury. Together, these studies indicate a novel role for MHC class I proteins in translating electrical activity into changes in synaptic strength and neuronal connectivity in vivo.
Keywords: hippocampus, LTP, LTD, immune, remodeling
INTRODUCTION
The major histocompatibility complex (MHC) class I is a large family of vertebrate genes that was discovered as the molecular basis for the rejection of grafted tissue. Subsequently, it was found that MHC class I plays a much broader role in the discrimination of self from non-self during bacterial and viral infections. MHC class I is expressed on the surface of most nucleated cells in the body, where it presents antigens derived from cytosolic proteins. Stabilizing these antigens at the cell surface enables interactions with antigen-specific cytotoxic T cells, which subsequently kill infected cells. The ability to quickly and accurately identify and dispatch cellular sources of foreign proteins is one of the central features of the adaptive immune response.
Surprisingly, growing evidence indicates that these immune functions are only the tip of the iceberg, and that MHC class I proteins also perform crucial roles outside the immune system, specifically in activity-dependent development and plasticity of the vertebrate nervous system. In this review, I summarize recent studies that characterize MHC class I expression and function in the nervous system, and discuss what these studies suggest about the relationship between MHC class I and activity-dependent structural and functional plasticity.
Expression of MHC class I in neurons
It has long been known that neurons and glia can be induced to express MHC class I in the brain after challenges that include injury, infection and treatment with cytokines (Fisher, 1984; Lampson and Fisher, 1984; Wong et al., 1984; Maehlen et al., 1988; Streit et al., 1989; Pereira et al., 1994; Neumann et al., 1995; Fujimaki et al., 1996; Neumann et al., 1997; Redwine et al., 2001; Foster et al., 2002). However, neurons were generally thought not to express MHC class I in the resting state (Lampson and Fisher, 1984; Lampson et al., 1988; Joly et al., 1991; Joly and Oldstone, 1992; Drew et al., 1993; White et al., 1994; Lampson, 1995; Fujimaki et al., 1996). This is supported by the fact that some viruses persist in the CNS compared with other tissues (Joly et al., 1991; Rall et al., 1995) and that tissue grafts into the brain are not rejected immediately, as they are elsewhere (Head and Griffin, 1985). These functional properties earned the CNS the label of ‘immunologically privileged’.
However, several studies have shown since that MHC class I is expressed in normal, uninjured neurons (Corriveau et al., 1998; Lidman et al., 1999; Linda et al., 1999; Huh et al., 2000) (Fig. 1). MHC class I mRNA and/or protein have been detected in subsets of normal neurons including developing lateral geniculate nucleus (LGN) neurons, developing and adult hippocampal neurons, primary somatosensory cortex, layer IV of the primary visual cortex, hippocampal pyramidal cells (Corriveau et al., 1998; Huh et al., 2000), substantia nigra pars compacta (Lidman et al., 1999), dorsal root ganglion cells, adult brainstem and spinal motoneurons (Linda et al., 1999), and sensory neurons of the vomeronasal organ (Ishii et al., 2003; Loconto et al., 2003). In parallel, it has been shown that the immune privilege of the brain is likely to result from modified responses to MHC class I–peptide complexes in the CNS, rather than a lack of MHC class I proteins on neurons (reviewed in Streilein, 1993).
Fig. 1. Expression of three MHC class I mRNAs in a coronal section of adult mouse brain.
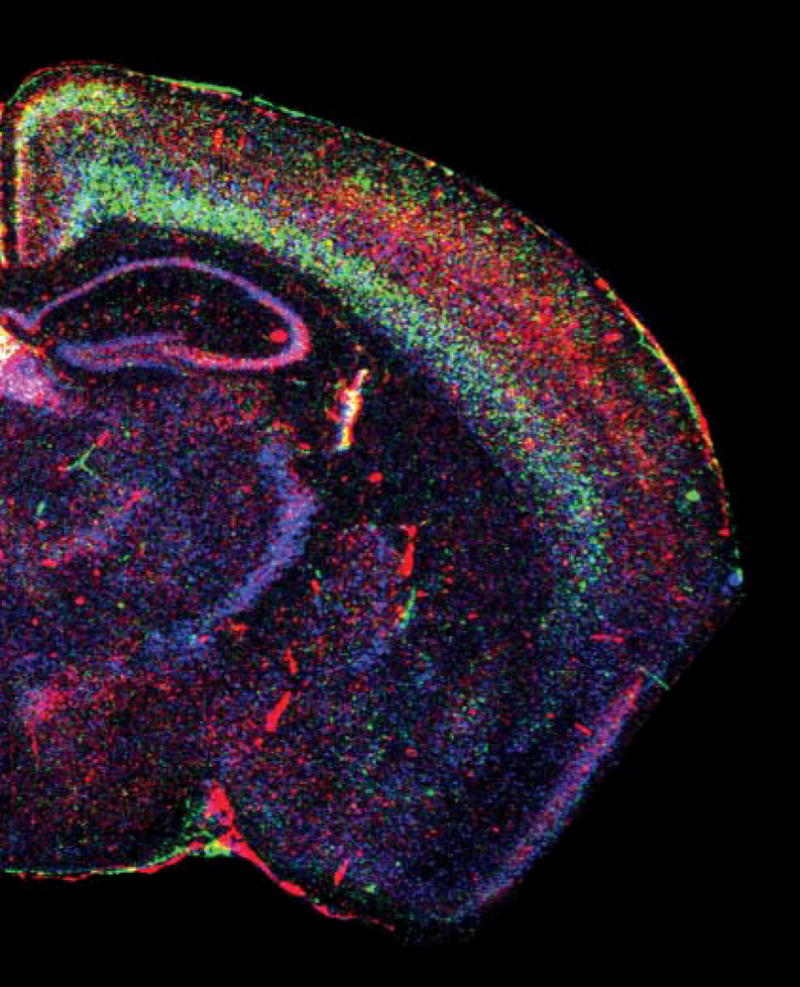
Pseudocolored in situ hybridization using probes against the classical MHC class I H-2D (blue), and the non-classical MHC class Is T22 (red) and Qa-1 (green). Reprinted with permission from Huh et al. (2000).
Although MHC class I is detected in neurons, its expression in the brain differs in many ways from that seen in most other nucleated cells. First, the concentration of MHC class I in neurons is lower than in many other cell types (Corriveau et al., 1998; Huh et al., 2000). Lower MHC class I levels might reduce the risk of autoimmunity (Singer, 1997) in the brain, where individual cells are relatively difficult to replace. Second, MHC class I is expressed in most tissues at relatively constant levels in the absence of infection and other challenge (Janeway et al., 2001). By contrast, MHC class I levels in the brain are highly dynamic, even in the absence of infection and injury, and the levels and localization of MHC class I mRNAs change dramatically during normal development (Corriveau et al., 1998; Huh et al., 2000). Third, some so-called ‘non-classical’ MHC class I proteins are not detected in other tissues but are strongly (and in some cases exclusively) expressed in specific regions of the CNS. For example, members of the M10 family of non-classical MHC class Is have been detected only in the vomeronasal organ, a specialized pheromone-sensing olfactory structure (Ishii et al., 2003; Loconto et al., 2003). Last, most antigen-presenting cells co-dominantly express all of the classical MHC class I alleles in an individual’s genome (Janeway et al., 2001), but it is unknown if a given CNS neuron expresses the full array of classical MHC class I proteins. However, members of the non-classical MHC class I M10 family are expressed in subpopulations of sensory neurons in the VNO, such that each neuron expresses either one or a few of these non-classical MHC class I molecules (Ishii et al., 2003; Loconto et al., 2003). Furthermore, in situ hybridization reveals overlapping but distinct patterns of mRNA expression for several different classical and non-classical MHC class Is (Fig. 1) (Huh et al., 2000), which indicates that different neurons express different compliments of MHC class I proteins. It is, therefore, of interest to examine the expression patterns of the many members of the classical and non-classical MHC class I families in the brain, to ascertain whether there is a novel molecular logic to their neuronal expression.
The sub-cellular localization of neuronal MHC class I is also unusual compared with other cell types, most of which express MHC class I over their entire surface. In several brain regions, neuronal MHC class I proteins occur selectively in the somato–dendritic compartment. For example, in the retinogeniculate and geniculocortical systems, MHC class I is high in neurons postsynaptic to actively remodeling axons (Corriveau et al., 1998). In acutely dissociated hippocampal neurons in vitro, MHC class I protein is detected throughout dendrites (Goddard and Shatz, 2003). Similarly, in acutely dissociated cortical neurons in vitro, MHC class I protein is detected initially in clusters in both axons and dendrites, but becomes localized selectively to dendrites as the cultures age (Wampler and McAllister, 2004). In these cells, MHC class I is detected at both excitatory and inhibitory synapses (Wampler and McAllister, 2004).
In the somatodendritic compartment, some of MHC class I occurs at synapses. Immunostaining of hippocampal neurons in vitro shows MHC class I throughout the dendrites, including some that is localized to spines and apposed to presynaptic markers (Goddard and Shatz, 2003). Furthermore, MHC class I immunoreactivity is enriched in synaptosomal preparations of whole adult rat brain (Huh et al., 2000).
Interestingly, the regional pattern of MHC class I expression in the brain correlates with sites of ongoing activity-dependent plasticity, including the developing visual system, and adult hippocampus and cerebellum (Corriveau et al., 1998; Huh et al., 2000). Protein and/or mRNA that encodes several MHC class I receptors, including CD3ζ (Corriveau et al., 1998; Huh et al., 2000), TCR beta (Syken and Shatz, 2003a), Digr1, Digr2 (Syken and Shatz, 2003b) and KIR (Bryceson et al., 2005), are also found in the brain, and most are expressed in highly plastic regions of the CNS. Thus, the expression patterns of both MHC class I and candidate MHC class I receptors are consistent with a role for MHC class I signaling in activity-dependent plasticity in the adult and developing brain (Corriveau et al., 1998; Huh et al., 2000; Boulanger et al., 2001).
Electrical activity regulates neuronal expression of MHC class I
One expectation of genes that are required for activity-dependent plasticity is that at least a subset of them is regulated by the relevant electrical activity (Corriveau et al., 1998). In many parts of the developing brain, spontaneous electrical activity drives the establishment of the final, adult pattern of connections. In the developing mammalian visual system, where this process is relatively well-understood, each eye sends axons to a proximal target in the thalamus, the LGN. Initially, these inputs are overlapping, but later in development they segregate into eye-specific layers, through a process that requires electrical activity arising in the retinas. To identify genes involved in activity-dependent remodeling, Corriveau et al. 1998 performed an unbiased genetic screen for LGN-expressed genes that are regulated by endogenous electrical activity during this crucial period in development. Unexpectedly, this screen showed that a member of the MHC class I family is downregulated in the absence of the normally occurring spontaneous electrical activity that drives remodeling of afferents in the developing LGN (Corriveau et al., 1998). This initial finding spurred further characterization of the expression patterns, regulation (above) and effects (below) of MHC class I in the CNS.
In addition to early spontaneous activity, MHC class I is also unregulated by later, visually driven activity (Corriveau et al., 1998). In addition, MHC class I can be upregulated by exogenous activity. MHC class I levels increase in the adult hippocampus and neocortex after kainic acid-induced seizures, which dramatically increase activity (Corriveau et al., 1998).
Thus, in many situations in vivo MHC class I is upregulated by electrical activity and downregulated when activity is reduced (Corriveau et al., 1998). By contrast, dissociated hippocampal neurons in vitro upregulate MHC class I in response to interferon γ (IFN-γ) only under electrically silenced conditions (Neumann et al., 1995; Neumann et al., 1997). There are many possible explanations for these differing results. One is that the experiments are not comparable because of the cell type and system used; some experiments were performed on dissociated hippocampal neurons in vitro (Neumann et al., 1995; Neumann et al., 1997) whereas others examined several neuronal populations in vivo (Corriveau et al., 1998; Huh et al., 2000). Furthermore, recent preliminary studies indicate that one crucial feature might be whether the activity blockade is either local or global. In dissociated cortical neurons in vitro, global depression of activity via bath application of TTX increases the density of MHC class I immunoreactivity (Wampler and McAllister, 2004). This is reminiscent of previous results showing that MHC class I is upregulated in dissociated hippocampal neurons in vitro in response to treatment with IFN-γ, but only if activity is globally blocked with TTX (Neumann et al., 1995; Neumann et al., 1997). However, when activity is blocked in a single cell (through over-expression of a gene encoding a channel that clamps the voltage of the cell at a hyperpolarized resting potential), activity blockade instead decreases the expression of MHC class I (Wampler and McAllister, 2004), which is consistent with earlier results in vivo (Corriveau et al., 1998). Thus, MHC class I expression might be differentially regulated by activity depending on the cell type, local network properties and activity levels, and the relative level of activity in neighboring cells. Given this possibility, it would be interesting to know if more subtle modulation of the level and pattern of activity of a single neuron is also a relevant signal for the regulation of MHC class I expression. This might be the case if MHC class I is a mediator of plasticity, because many forms of activity-dependent plasticity are only induced by specific patterns of electrical activity.
Together, these results indicate that electrical activity can bi-directionally regulate the expression of MHC class I in a given neuron or population of neurons. This regulation can occur either prenatally or postnatally, and in response to either spontaneous or sensory-evoked endogenous activity as well as exogenous activity (seizure). In addition, the circuit activity and relative levels of activity in neighboring neurons might contribute to the extent and even the direction of the change in MHC class I levels in a given neuron.
MHC class I in remodeling of developing and adult connections
MHC class I was identified in a blind screen for genes that are involved in developmental activity-dependent remodeling (Corriveau et al., 1998), is expressed highly in regions of ongoing plasticity, and is regulated by activity (Corriveau et al., 1998; Huh et al., 2000). Together, these results indicate that MHC class I might be involved in activity-dependent plasticity (Corriveau et al., 1998; Huh et al., 2000; Boulanger et al., 2001; Boulanger and Shatz 2004). To test this hypothesis directly, Huh et al. 2000 examined activity-dependent structural plasticity in mice that are genetically deficient for cell-surface MHC class I. These mice are genetically deficient for β2-microglobulin (β2m), the obligatory light chain that associates with many MHC class I heavy chains, and TAP1, a transporter that is required to load intracellular peptides onto many MHC class I molecules. In the absence of both of these products, cell-surface concentrations of MHC class I are greatly reduced (Zijlstra et al., 1990; Jackson and Peterson, 1993; Dorfman et al., 1997; Neumann et al., 1997). In addition, Huh et al. examined mice deficient for CD3ζ, a component of many known receptors for MHC class I in the immune system (Love et al., 1993). These mice are immune compromised, but are outwardly normal if kept in a clean facility, and their gross neuroanatomy is normal (Huh et al., 2000). All experiments on these mice discussed below were performed blind to genotype.
Because MHC class I was identified in a screen for genes that are involved in the remodeling of the developing visual system, and MHC class I is expressed highly in the developing LGN during the period when eye-specific layers are forming, Huh et al. examined visual system development in MHC class I-deficient mice. In mice deficient for MHC class I signaling, the final, activity-dependent steps in the remodeling of visual projections to the LGN are selectively disrupted (Huh et al., 2000). Although the inputs from the two eyes arrive in their appropriate target, the LGN, they do not form the tightly localized, eye-specific regions seen in wild-type mice. The projections from one eye to the ipsilateral LGN are significantly larger in MHC class I-deficient mice, and aberrant, ectopic projections occur. These results are consistent with a role for endogenous MHC class I in the removal of inappropriate connections during normal development (Huh et al., 2000; Boulanger, 2001; Boulanger, 2004. Furthermore, the fact that CD3ζ-deficient mice are indistinguishable from MHC class I-deficient mice with regard to retinogeniculate remodeling defects indicates that MHC class I might signal through a CD3ζ-containing receptor in the developing brain, as it does in the immune system (Huh et al., 2000) (see below).
Activity drives the remodeling of many other developing connections outside the visual system. For instance, during motor development, there is selective elimination of innervation of autonomic postganglionic neurons, skeletal muscle fibers, and somatic motoneurons (Edstrom et al., 2004). This remodeling of motor connections, like remodeling of the developing visual system, is driven by electrical activity, which indicates that they might share significant mechanistic features. But if MHC class I is required for widespread neuro-developmental remodeling, why are the brains of MHC class I-deficient mice grossly normal? This is, perhaps, unsurprising because although activity-dependent refinement is common, in most regions it is the final stage of a long developmental process. Many early developmental events, including neuronal-cell division, migration, path finding, and the establishment of coarse mapping, proceed as usual in the absence of activity. Thus, each of these stages of gross development might be expected to proceed normally in MHC class I-deficient mice.
In addition to its role in normal developmental activity-dependent remodeling of neuronal connectivity, MHC class I is also implicated in pathological remodeling, as both a consequence of normal aging and in response to acute injury. MHC class I is upregulated in normal, aged motoneurons (Edstrom et al., 2004) at a time when there is a pronounced, progressive loss of afferent boutons. Thus, it is possible that this pathological retraction of projections, like the normal developmental regression of inappropriate visual projections, is mediated by MHC class I.
Neurons also upregulate MHC class I in response to axotomy (Olsson et al., 1989; Linda et al., 1998) and infection (Foster et al., 2002), both of which can induce selective withdrawal of affected synaptic terminals from cell bodies and dendrites. In the facial nucleus, peripheral nerve transection leads to pruning of synapses from the affected neuronal bodies. Similarly, transection of spinal motor nerves leads to a reduction in the number of synapses in the relevant spinal cord areas. Interestingly, mice that lack MHC class I exhibit greater synapse reduction after transfection, and this aberrant pruning affects inhibitory synapses more severely (Oliveira et al., 2004). This implies that endogenous MHC class I acts at these synapses to either reduce degeneration or enhance regeneration of inhibitory terminals. These results are in apparent contrast to those in the CNS, where endogenous MHC class I increases pruning of inappropriate connections. It is likely that these apparent differences reflect mechanistic differences between normal developmental processes and responses to injury, as well as the diverse roles of MHC class I, which depend on cell type and developmental age. Together, these observations indicate that endogenous MHC class I participates in the remodeling of diverse synaptic connections, at different ages, under both normal and pathological conditions, in the CNS and PNS.
MHC class I in activity-dependent synaptic plasticity
How is remodeling accomplished on a cellular level? One hypothesis is that changes in the strength of individual synapses, such as potentiation and depression, lead to stabilization and withdrawal, respectively, of the affected connections. This hypothesis predicts that MHC-deficient mice, which lack normal activity-dependent remodeling, might also have defects in activity-dependent synaptic plasticity. To test this directly, Huh et al. examined synaptic plasticity at CA3–CA1 synapses in the adult hippocampus, where the cellular mechanisms of synaptic plasticity are relatively well-understood, and where MHC class I is expressed strongly (Corriveau et al., 1998; Huh et al., 2000). Unexpectedly, they found that long-term potentiation (LTP) is enhanced significantly at these synapses in MHC class I-deficient mice (Fig. 2). This enhancement of LTP appears to be caused by amplification of existing plasticity mechanisms, because it is fully blocked by NMDAR antagonists in both wild-type and MHC-deficient mice. In addition, MHC-deficient mice lack long-term depression (LTD), and synaptic plasticity is systematically shifted in favor of potentiation across a wide range of stimulation frequencies (Fig. 2). As in the developing retinogeniculate projection, CD3ζ-deficient mice are phenocopies of MHC class I-deficient mice in the adult hippocampus (Fig. 2), which indicates either a direct or indirect role for a CD3ζ-containing receptor in activity-dependent synaptic plasticity in the hippocampus (Huh et al., 2000).
Fig. 2. MHC class I signaling is required for normal bi-directional synaptic plasticity in the adult hippocampus.
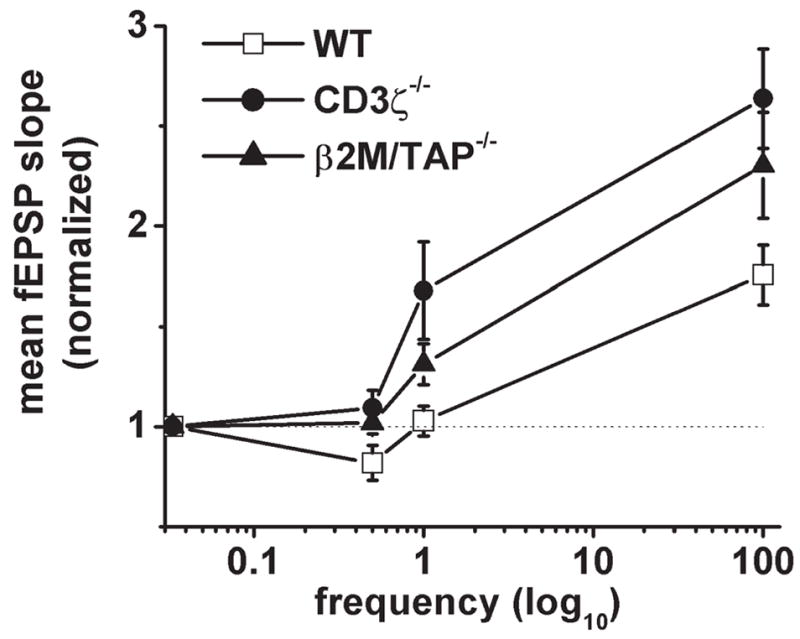
In wild-type adult mice, low-frequency stimulation leads to long-lasting synaptic depression, whereas higher frequencies result in potentiation. In mice deficient for either MHC class I or CD3ζ, the frequency-response curve shifts systematically in favor of potentiation. Reprinted with permission from Huh et al. (2000).
These changes in potentiation and depression are not caused by altered inhibition in the absence of MHC class I because they persist in the presence of GABAA receptor blockers. Importantly, these changes in synaptic plasticity are also not an indirect effect of immune compromise, because more severely immunocompromised RAG1-deficient mice have normal plasticity at these synapses (Huh et al., 2000). Although most knockout mice exhibit loss of LTP, rather than enhancement, a few other mutant mice also exhibit enhanced LTP and no LTD at these synapses. These include mice mutant for PSD-95 (Migaud et al., 1998) and cadherin-11 (Manabe et al., 2000). Like these proteins, MHC class I is required for normal, bi-directional, synaptic plasticity in the adult hippocampus (Huh et al., 2000; Boulanger et al., 2001; Boulanger and Shatz, 2004). It is important to determine the cellular and molecular mechanisms whereby MHC class I affects hippocampal plasticity. It is also of interest to characterize the rules of normal synaptic plasticity at the developing retinogeniculate synapse and to examine if functional alterations in plasticity at these synapses precede the failure of visual remodeling in MHC-deficient mice.
Mechanisms of activity dependence
As yet, it is unclear why MHC class I seems to be involved in diverse activity dependent events in brain development, plasticity, and response to injury. One possibility is that the relatively low basal levels of MHC class I in neurons combined with its upregulation by activity restricts its action to periods of unusually high electrical activity. For example, the electrical activity that drives the final refinement of retinal projections upregulates the expression of MHC class I in the LGN, and MHC acts to promote removal of inappropriate connections in the LGN at this time (Corriveau et al., 1998). In this model, MHC class I concentrations function as a molecular readout of activity levels. Interestingly, a component of many MHC class I receptors in the immune system, CD3ζ, is also expressed in neurons in the developing and adult brain, including the developing LGN and adult hippocampus (Corriveau et al., 1998; Huh et al., 2000), but its concentration is not regulated by spontaneous activity in the developing LGN (Corriveau et al., 1998). Similarly, the light chain of many MHC class Is, β2m, is not upregulated in response to increased activity (Corriveau et al., 1998). Thus, it is possible that MHC class I co-subunits and receptors are present normally at or near the synapse, and that MHC class I heavy-chain abundance is limiting and confers activity-dependence to its signaling.
However, some of the abnormal responses to activity in MHC class I-deficient mice occur within minutes (e.g. altered hippocampal synaptic plasticity), before activity-driven transcription and translation of MHC class I protein are likely to be significant. In these cases, it might be that the lack of MHC class I affects earlier events in brain development that change the starting state of the synapse. Alternatively, synapses in MHC-deficient mice might be indistinguishable initially from wild type, but might be unable to engage MHC class I-dependent mechanisms at the time of plasticity induction. The question of whether MHC class I acts early in development, throughout life, or acutely, for example, in the presence of plasticity-inducing patterns of activity, is a pressing topic for future study.
One interesting implication of the above results is that MHC class I might potentially participate in a negative-feedback loop involving neuronal activity (Goddard and Shatz, 2003). For example, in the adult hippocampus, elevated activity can increase expression of MHC class I (Corriveau et al., 1998). In turn, endogenous MHC class I at these synapses limits the magnitude of LTP and permits LTD (Huh et al., 2000). Thus neurons with high activity levels will express more MHC class I and, as a result, their plasticity might shift in favor of synaptic weakening, perhaps reducing their activity and maintaining them within a functional dynamic range. Conversely, less-active neurons might downregulate MHC class I, which in the hippocampus shifts the plasticity balance in favor of potentiation. In this way, MHC class I may be important in the regulation of synaptic strength at these and other synapses. Again, such homeostatic adjustments might occur either early in development, permanently changing the ‘set point’ of adult synapses, or acutely, as the levels of activity at adult synapses change in response to experience.
MHC class I signaling in neurons
MHC class I is expressed in the somatodendritic compartment in the developing retinogeniculate and geniculocortical projections (Corriveau et al., 1998; Huh et al., 2000), and in hippocampal neurons in culture in spines that are apposed to sites of presynaptic contact (Goddard and Shatz, 2003). However, evidence from mice deficient in MHC class I reveals changes in presynaptic structures at these same synapses, including aberrant remodeling of retinal ganglion cell arbors (Huh et al., 2000) and changes in presynaptic ultrastructure in hippocampal neurons (Goddard and Shatz, 2003). These data indicate that post-synaptically expressed MHC class I induces retrograde signaling to affect presynaptic structures (Fig. 3).
Fig. 3. MHC class I signaling might involve retrograde signaling.
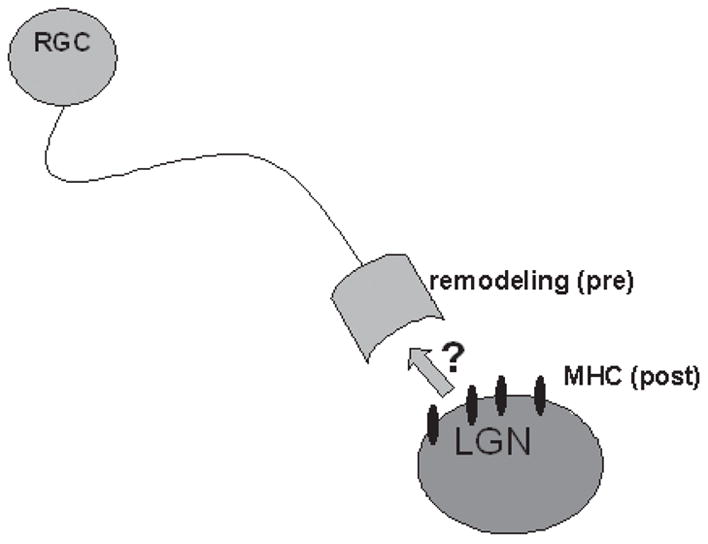
In the developing visual system and other regions of the developing and adult brain (see text), MHC class I is expressed in the somatodendritic compartment. In the absence of normal MHC class I signaling, remodeling of retinal ganglion cell (RGC) axons, which are pre-synaptic to LGN neurons that express MHC, is abnormal. This indicates that MHC class I might signal through an unknown retrograde messenger at these and other synapses.
How could such signaling be mediated? One possibility is that MHC class I engages signaling through traditional neuronal retrograde messengers, including nitric oxide and endocannabinoids. Alternatively, T-cell receptors and other classical immunoreceptors for MHC class I might be expressed by presynaptic neurons (Boulanger et al., 2001; Syken and Shatz, 2003a; Syken and Shatz, 2003b). Indeed, several recent studies find evidence for mRNA and/or protein encoding components of numerous known immunoreceptors in neurons (Corriveau et al., 1998; Huh et al., 2000; Syken and Shatz, 2003a; Syken and Shatz, 2003b; Bryceson et al., 2005). Downstream signaling molecules for these receptors also occur in neurons, some of which (including Grb-2 and fyn) are localized to the presynaptic terminal. Interestingly, many of these downstream immune signaling molecules also have known roles in activity-dependent plasticity in the CNS (reviewed in Boulanger et al., 2001).
CONCLUSIONS
MHC class I is expressed by normal neurons throughout life. Expression of MHC class I is regulated by the naturally occurring electrical activity that sculpts developing projections. In the adult, MHC class I expression is primarily dendritic and remains sensitive to both natural and pathological changes in activity. Several independent studies have determined that MHC class I is required for normal activity dependent potentiation, depression, removal of inappropriate connections, and responses to injury in the CNS and PNS. Thus, MHC class I is crucial for translating activity into changes in synaptic strength and neuronal connectivity in vivo.
Acknowledgments
I would like to acknowledge Carla Shatz, in whose lab many of these experiments were performed. I would also like to thank Gene Huh, Roderick Corriveau, Hongping Du, Tilmann Brotz, and Patricio Riquelme, all members of the Shatz Laboratory who contributed to the work described here. The author’s work described in this review was supported by grant 1F32EY07016 from the National Institutes of Health and a Junior Fellowship from the Harvard Society of Fellows to L.M.B.
References
- Boulanger LM, Huh GS, Shatz CJ. Neuronal plasticity and cellular immunity: shared molecular mechanisms. Current Opinion in Neurobiology. 2001;11:568–578. doi: 10.1016/s0959-4388(00)00251-8. [DOI] [PubMed] [Google Scholar]
- Boulanger LM, Shatz CJ. Immune signalling in neural development, synaptic plasticity and disease. Nature Reviews Neuroscience. 2004;5:521–531. doi: 10.1038/nrn1428. [DOI] [PubMed] [Google Scholar]
- Bryceson YT, Foster JA, Kuppusamy SP, Herkenham M, Long EO. Expression of a killer cell receptor-like gene in plastic regions of the central nervous system. Journal of Neuroimmunology. 2005;161:177–182. doi: 10.1016/j.jneuroim.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Corriveau RA, Huh GS, Shatz CJ. Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron. 1998;21:505–520. doi: 10.1016/s0896-6273(00)80562-0. [DOI] [PubMed] [Google Scholar]
- Dorfman JR, Zerrahn J, Coles MC, Raulet DH. The basis for self-tolerance of natural killer cells in beta2-microglobulin- and TAP-1- mice. Journal of Immunology. 1997;159:5219–5225. [PubMed] [Google Scholar]
- Drew PD, Lonergan M, Goldstein ME, Lampson LA, Ozato K, McFarlin DE. Regulation of MHC class I and beta 2-microglobulin gene expression in human neuronal cells. Factor binding to conserved cis-acting regulatory sequences correlates with expression of the genes. Journal of Immunology. 1993;150:3300–3310. [PubMed] [Google Scholar]
- Edstrom E, Kullberg S, Ming Y, Zheng H, Ulfhake B. MHC class I, beta2 microglobulin, and the INF-gamma receptor are upregulated in aged motoneurons. Journal of Neuroscience Research. 2004;78:892–900. doi: 10.1002/jnr.20341. [DOI] [PubMed] [Google Scholar]
- Foster JA, Quan N, Stern EL, Kristensson K, Herkenham M. Induced neuronal expression of class I major histocompatibility complex mRNA in acute and chronic inflammation models. Journal of Neuroimmunology. 2002;131:83–91. doi: 10.1016/s0165-5728(02)00258-8. [DOI] [PubMed] [Google Scholar]
- Fujimaki H, Hikawa N, Nagoya M, Nagata T, Minami M. IFN-gamma induces expression of MHC class I molecules in adult mouse dorsal root ganglion neurones. Neuroreport. 1996:2951–2955. doi: 10.1097/00001756-199611250-00030. [DOI] [PubMed] [Google Scholar]
- Goddard CA, Shatz CJ. Role for class I MHC in regulating presynaptic structure. 2003 Abstract Viewer: Society For Neuroscience 2003 Program 784.10. [Google Scholar]
- Head JR, Griffin WS. Functional capacity of solid tissue transplants in the brain: evidence for immunological privilege. Proceedings of the Royal Society of London B Biological Science. 1985;224:375–387. doi: 10.1098/rspb.1985.0039. [DOI] [PubMed] [Google Scholar]
- Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T, Hirota J, Mombaerts P. Combinatorial coexpression of neural and immune multigene families in mouse vomeronasal sensory neurons. Current Biology. 2003;13:394–400. doi: 10.1016/s0960-9822(03)00092-7. [DOI] [PubMed] [Google Scholar]
- Jackson MR, Peterson PA. Assembly and intracellular transport of MHC class I molecules. Annual Reviews in Cell Biology. 1993;9:207–235. doi: 10.1146/annurev.cb.09.110193.001231. [DOI] [PubMed] [Google Scholar]
- Janeway CA, Travers P, Walport M, Shlomchik M, editors. Immunobiology. Garland Publishing; 2001. [Google Scholar]
- Joly E, Mucke L, Oldstone MB. Viral persistence in neurons explained by lack of major histocompatibility class I expression. Science. 1991;253:1283–1285. doi: 10.1126/science.1891717. [DOI] [PubMed] [Google Scholar]
- Joly E, Oldstone MB. Neuronal cells are deficient in loading peptides onto MHC class I molecules. Neuron. 1992;8:1185–1190. doi: 10.1016/0896-6273(92)90138-4. [DOI] [PubMed] [Google Scholar]
- Kim IJ, Beck HN, Lein PJ, Higgins D. Interferon gamma induces retrograde dendritic retraction and inhibits synapse formation. Journal of Neuroscience. 2002;22:4530–4539. doi: 10.1523/JNEUROSCI.22-11-04530.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampson LA, Fisher CA. Weak HLA and beta 2-microglobulin expression of neuronal cell lines can be modulated by interferon. Proceedings of the National Academy of Sciences of the USA. 1984;81:6476–6480. doi: 10.1073/pnas.81.20.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampson LA, Whelan JP, Siegel G. Functional implications of class I MHC modulation in neural tissue. Annals of the New York Academy of Sciences. 1988;540:479–482. doi: 10.1111/j.1749-6632.1988.tb27142.x. [DOI] [PubMed] [Google Scholar]
- Lampson LA. Interpreting MHC class I expression and class I/class II reciprocity in the CNS: reconciling divergent findings. Microscopy Research and Technique. 1995;32:267–285. doi: 10.1002/jemt.1070320402. [DOI] [PubMed] [Google Scholar]
- Lidman O, Olsson T, Piehl F. Expression of non-classical MHC class I (RT1-U) in certain neuronal populations of the central nervous system. European Journal of Neuroscience. 1999;11:4468–4472. doi: 10.1046/j.1460-9568.1999.00904.x. [DOI] [PubMed] [Google Scholar]
- Linda H, Hammarberg H, Cullheim S, Levinovitz A, Khademi M, Olsson T. Expression of MHC class I and beta2-microglobulin in rat spinal motoneurons: regulatory influences by IFN-gamma and axotomy. Experimental Neurology. 1998;150:282–295. doi: 10.1006/exnr.1997.6768. [DOI] [PubMed] [Google Scholar]
- Linda H, Hammarberg H, Piehl F, Khademi M, Olsson T. Expression of MHC class I heavy chain and beta2-microglobulin in rat brainstem motoneurons and nigral dopaminergic neurons. Journal of Neuroimmunology. 1999;101:76–86. doi: 10.1016/s0165-5728(99)00135-6. [DOI] [PubMed] [Google Scholar]
- Loconto J, Papes F, Chang E, Stowers L, Jones EP, Takada T, Kumanovics A, Fischer Lindahl K, Dulac C. Functional expression of murine V2R pheromone receptors involves selective association with the M10 and M1 families of MHC class Ib molecules. Cell. 2003;112:607–618. doi: 10.1016/s0092-8674(03)00153-3. [DOI] [PubMed] [Google Scholar]
- Love PE, Shores EW, Johnson MD, Tremblay ML, Lee EJ, Grinberg A, Huang SP, Singer A, Westphal H. T cell development in mice that lack the zeta chain of the T cell antigen receptor complex. Science. 1993;261:918–921. doi: 10.1126/science.7688481. [DOI] [PubMed] [Google Scholar]
- Maehlen J, Schroder HD, Klareskog L, Olsson T, Kristensson K. Axotomy induces MHC class I antigen expression on rat nerve cells. Neuroscience Letters. 1988;92:8–13. doi: 10.1016/0304-3940(88)90733-1. [DOI] [PubMed] [Google Scholar]
- Manabe T, Togashi H, Uchida N, Suzuki SC, Hayakawa Y, Yamamoto M, Yoda H, Miyakawa T, Takeichi M, Chisaka O. Loss of cadherin-11 adhesion receptor enhances plastic changes in hippocampal synapses and modifies behavioral responses. Molecular Cell Neuroscience. 2000;15:534–546. doi: 10.1006/mcne.2000.0849. [DOI] [PubMed] [Google Scholar]
- Migaud M, Charlesworth P, Dempster M, Webster LC, Watabe AM, Makhinson M, He Y, Ramsay MF, Morris RG, Morrison JH, O’Dell TJ, Grant SG. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature. 1998;396:433–439. doi: 10.1038/24790. [DOI] [PubMed] [Google Scholar]
- Neumann H, Cavalie A, Jenne DE, Wekerle H. Induction of MHC class I genes in neurons. Science. 1995;269:549–552. doi: 10.1126/science.7624779. [DOI] [PubMed] [Google Scholar]
- Neumann H, Schmidt H, Cavalie A, Jenne D, Wekerle H. Major histocompatibility complex (MHC) class I gene expression in single neurons of the central nervous system: differential regulation by interferon (IFN)-gamma and tumor necrosis factor (TNF)-alpha. Journal of Experimental Medicine. 1997;185:305–316. doi: 10.1084/jem.185.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira AL, Thams S, Lidman O, Piehl F, Hokfelt T, Karre K, Linda H, Cullheim S. A role for MHC class I molecules in synaptic plasticity and regeneration of neurons after axotomy. Proceedings of the National Academy of Sciences of the USA. 2004;101:17843–17848. doi: 10.1073/pnas.0408154101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson T, Kristensson K, Ljungdahl A, Maehlen J, Holmdahl R, Klareskog L. Gamma-interferon-like immunoreactivity in axotomized rat motor neurons. Journal of Neuroscience. 1989;9:3870–3875. doi: 10.1523/JNEUROSCI.09-11-03870.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira RA, Tscharke DC, Simmons A. Upregulation of class I major histocompatibility complex gene expression in primary sensory neurons, satellite cells, and Schwann cells of mice in response to acute but not latent herpes simplex virus infection in vivo. Journal of Experimental Medicine. 1994;180:841–850. doi: 10.1084/jem.180.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall GF, Mucke L, Oldstone MB. Consequences of cytotoxic T lymphocyte interaction with major histocompatibility complex class I-expressing neurons in vivo. Journal of Experimental Medicine. 1995;182:1201–1212. doi: 10.1084/jem.182.5.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redwine JM, Buchmeier MJ, Evans CF. In vivo expression of major histocompatibility complex molecules on oligodendrocytes and neurons during viral infection. American Journal of Pathology. 2001;159:1219–1224. doi: 10.1016/S0002-9440(10)62507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streilein JW. Immune privilege as the result of local tissue barriers and immunosuppressive microenvironments. Current Opinion in Immunology. 1993;5:428–432. doi: 10.1016/0952-7915(93)90064-y. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Graeber MB, Kreutzberg GW. Peripheral nerve lesion produces increased levels of major histocompatibility complex antigens in the central nervous system. Journal of Neuroimmunology. 1989;21:117–123. doi: 10.1016/0165-5728(89)90167-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syken J, Shatz CJ. Expression of T cell receptor beta locus in central nervous system neurons. Proceedings of the National Academy of Sciences of the USA. 2003a;100:13048–13053. doi: 10.1073/pnas.1735415100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syken J, Shatz CJ. Neuronal expression of immunoreceptors. 2003 Abstract Viewer: Society For Neuroscience 2003b Program 784.11. [Google Scholar]
- Wampler MS, McAllister KA. MHC class I protein is present and regulated by activity in dissociated cortical neurons during synaptogenesis. 2004 Abstract Viewer: Society For Neuroscience 2004 Program 614.5. [Google Scholar]
- White LA, Keane RW, Whittemore SR. Differentiation of an immortalized CNS neuronal cell line decreases their susceptibility to cytotoxic T cell lysis in vitro. Journal of Neuroimmunology. 1994;49:135–143. doi: 10.1016/0165-5728(94)90189-9. [DOI] [PubMed] [Google Scholar]
- Wong GH, Bartlett PF, Clark-Lewis I, Battye F, Schrader JW. Inducible expression of H-2 and Ia antigens on brain cells. Nature. 1984;310:688–691. doi: 10.1038/310688a0. [DOI] [PubMed] [Google Scholar]
- Zijlstra M, Bix M, Simister NE, Loring JM, Raulet DH, Jaenisch R. Beta 2-microglobulin deficient mice lack CD4-8+ cytolytic T cells. Nature. 1990;344:742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]


