Abstract
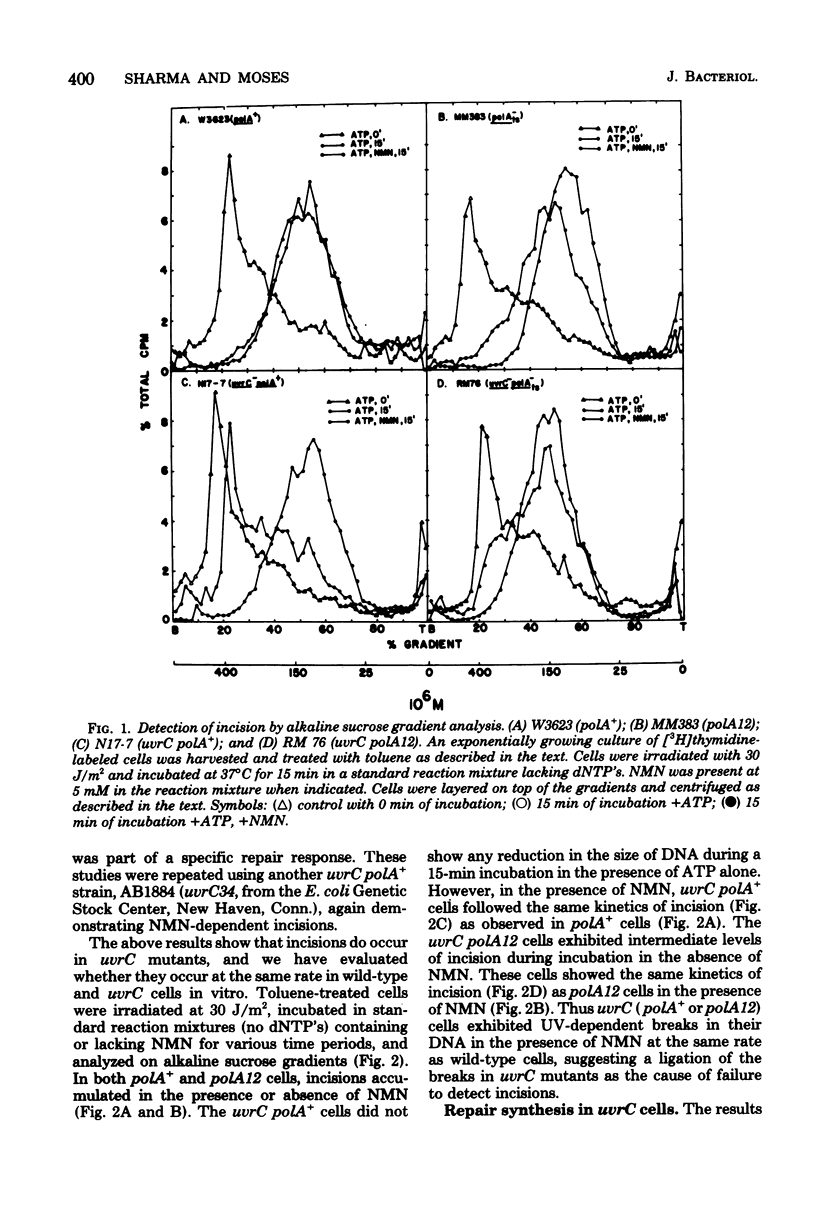
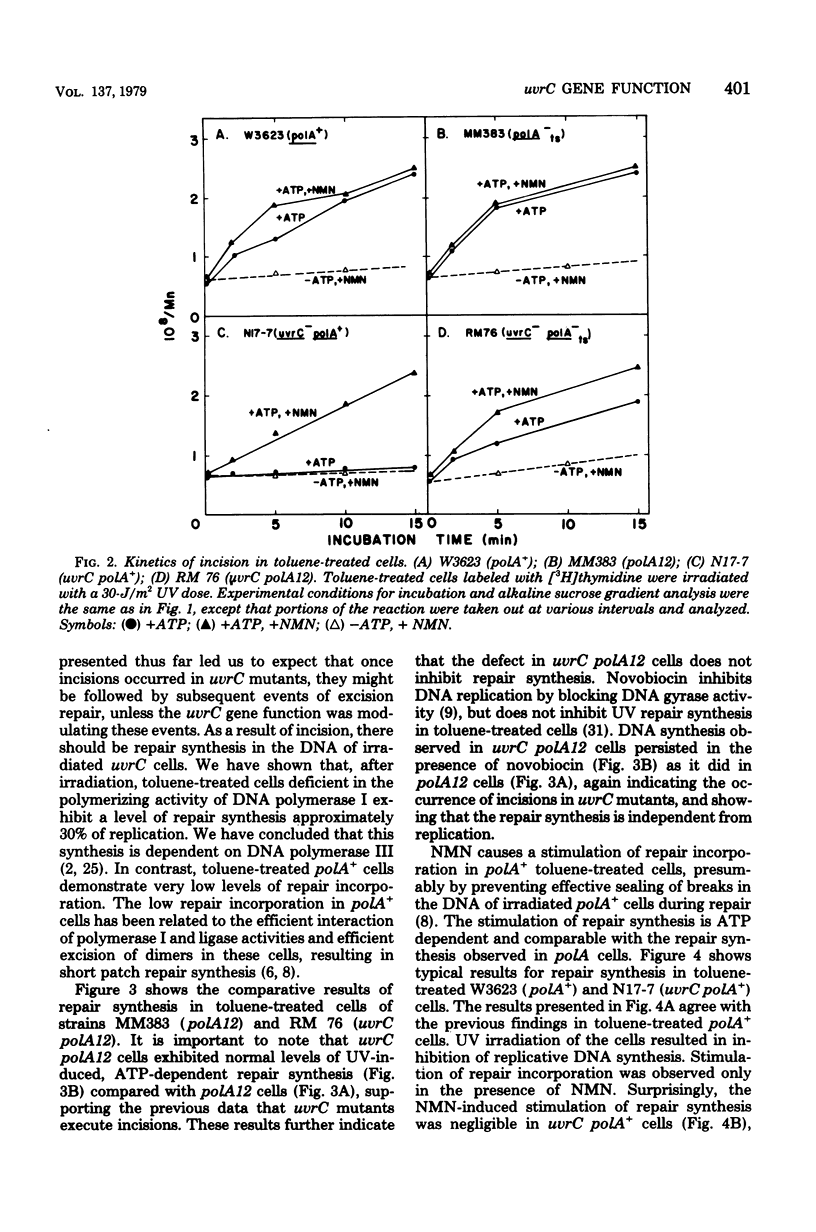
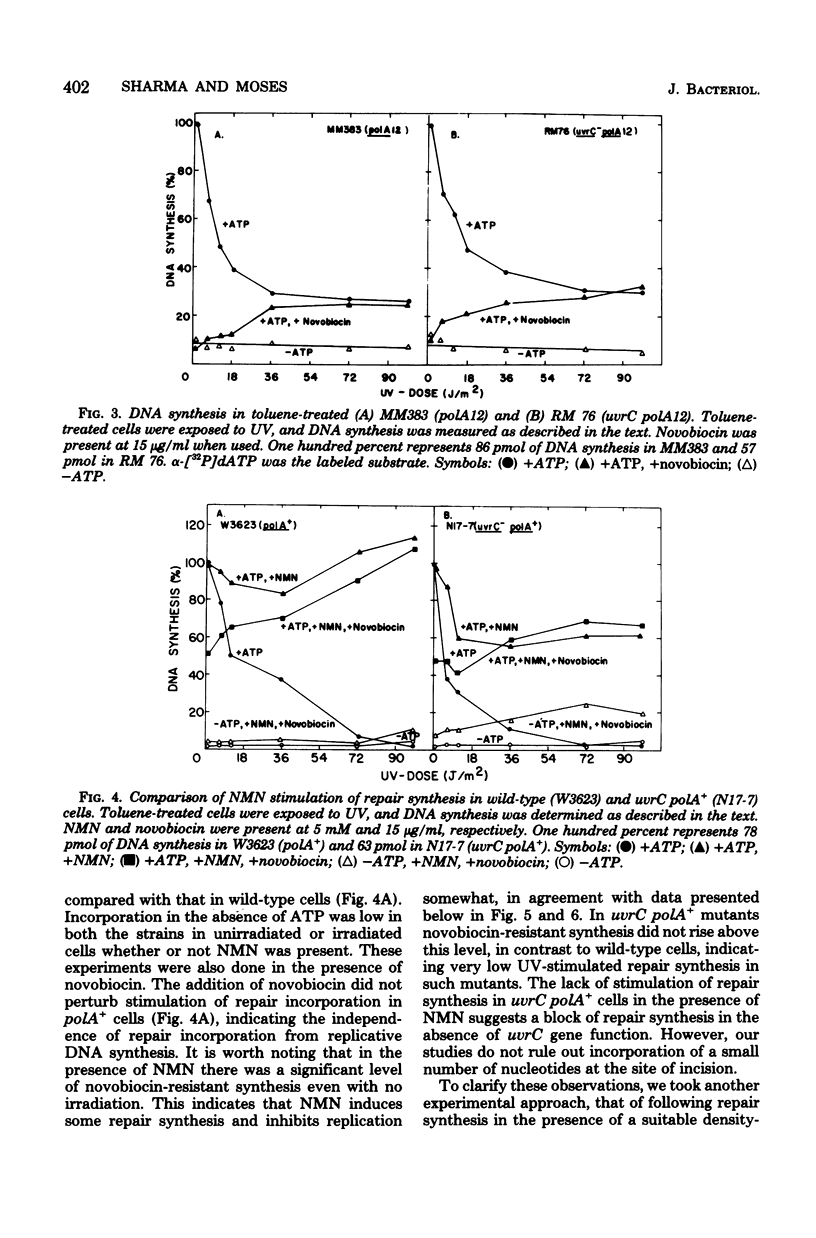
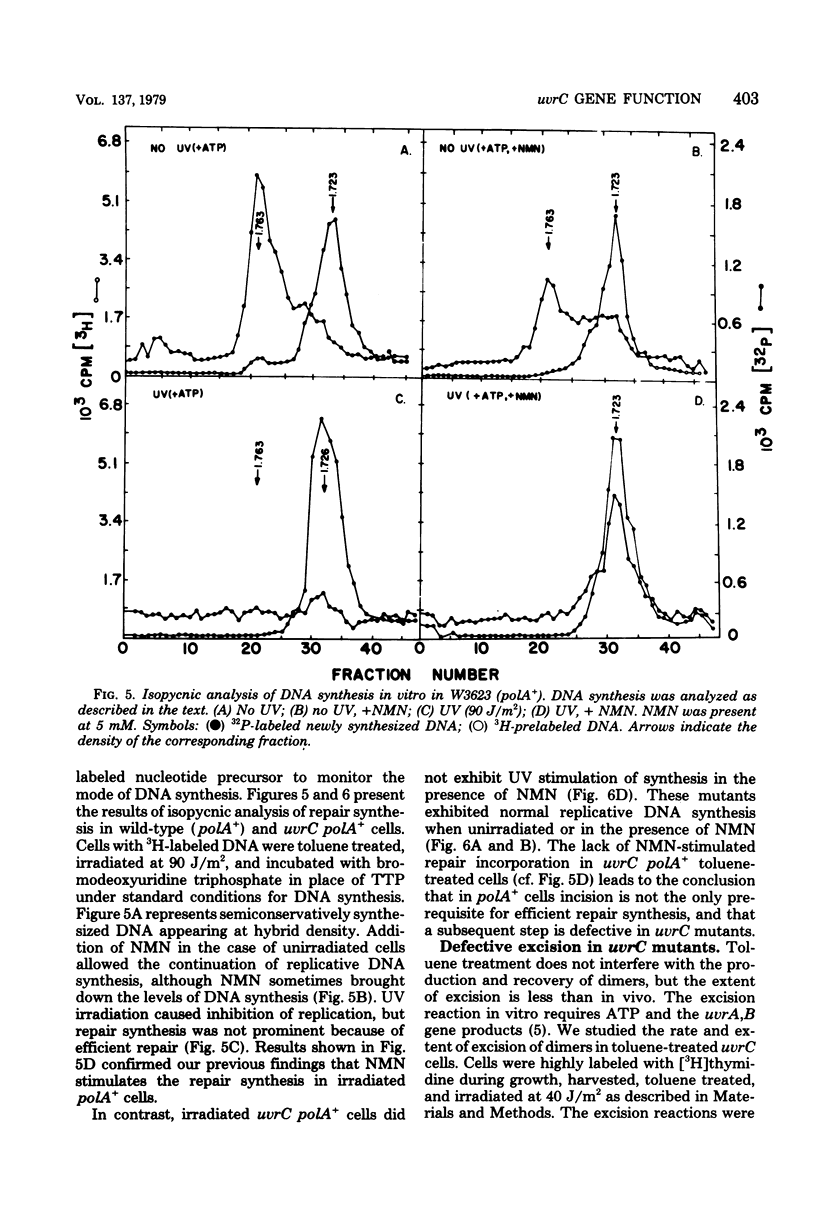
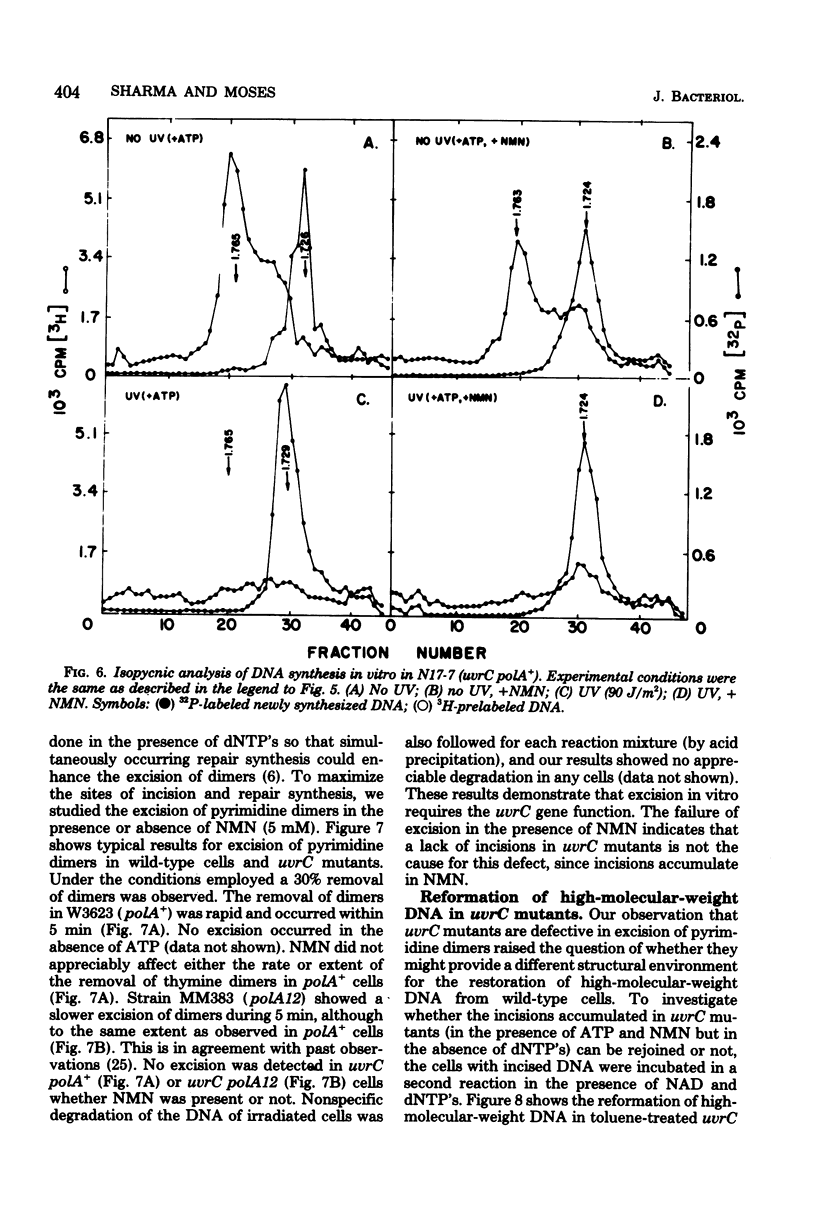
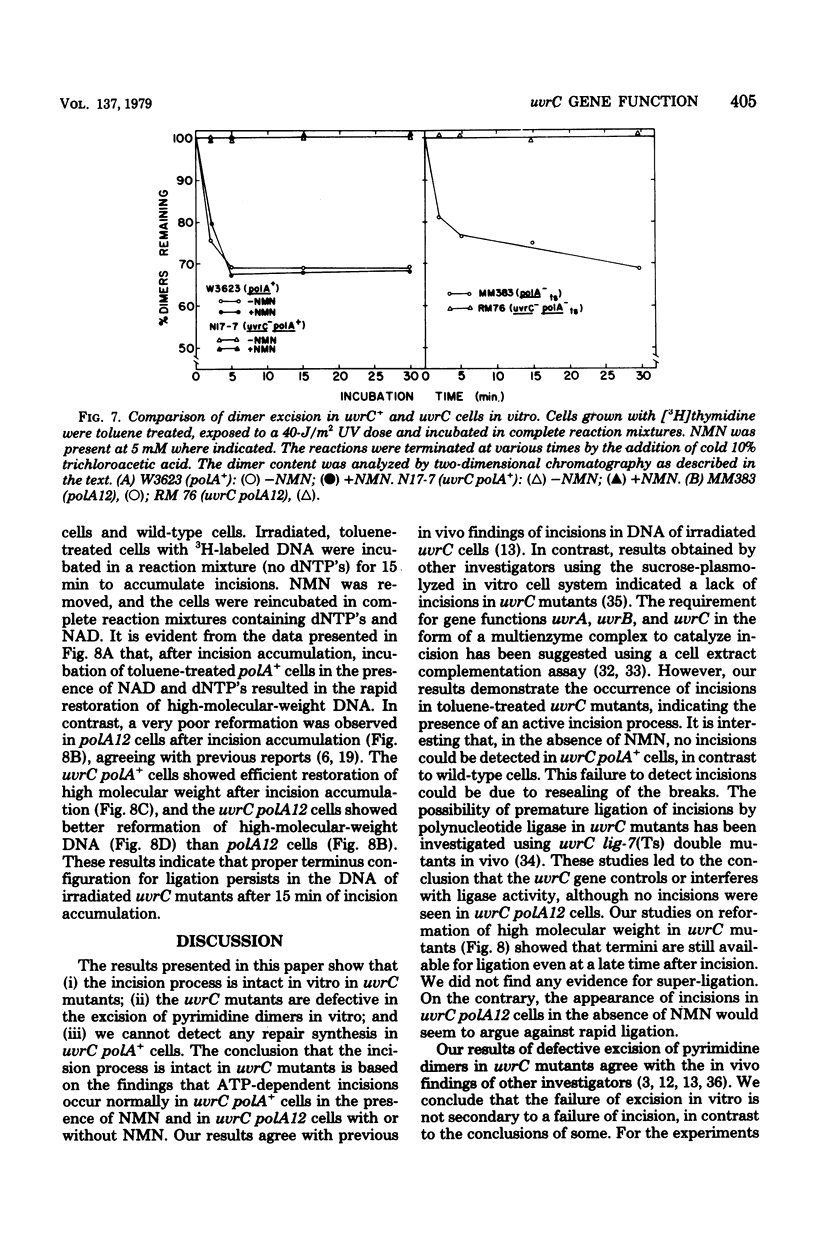
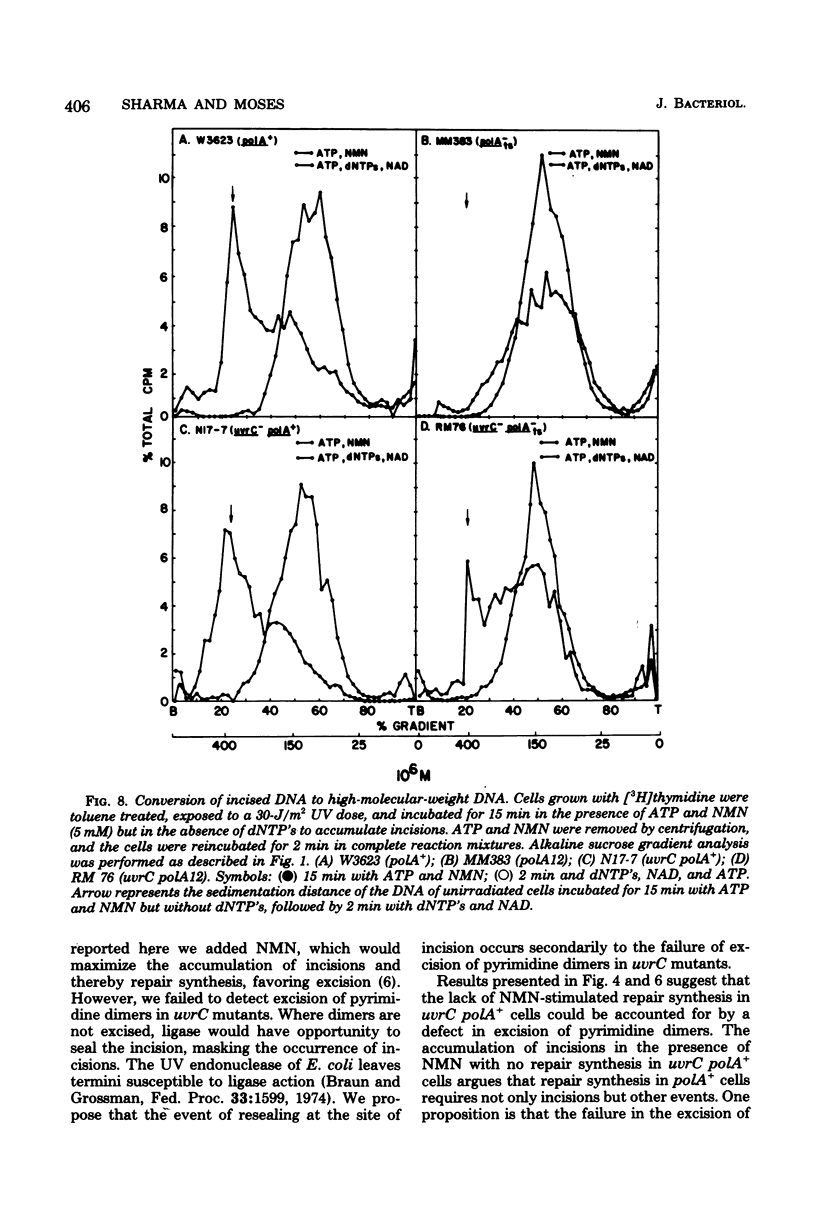
We have examined the role of the uvrC gene in UV excision repair by studying incision, excision, repair synthesis, and DNA strand reformation in Escherichia coli mutants made permeable to nucleoside triphosphates by toluene treatment. After irradiation, incisions occur normally in uvrC cells in the presence of nicotinamide mononucleotide (NMN), a ligase-blocking agent, but cannot be detected otherwise. We conclude that repair incisions are followed by a ligation event in uvrC mutants, masking incision. However, a uvrC polA12 mutant accumulates incisions only slightly less efficiently than a polA12 strain without NMN. Excision of pyrimidine dimers is defective in uvrC mutants (polA+ or polA12) irrespective of the presence or absence of NMN. DNA polymerase I-dependent, NMN-stimulated repair synthesis, which is demonstrable in wild-type cells, is absent in uvrC polA+ cells, but the uvrC polA12 mutant exhibits a UV-specific, ATP-dependent repair synthesis like parental polA12 strains. A DNA polymerase I-mediated reformation of high-molecular-weight DNA takes place efficiently in uvrC polA+ mutants after incision accumulation, and the uvrC polA12 mutant shows more reformation than the polA12 strain after incision. These results indicate that normal incision occurs in uvrC mutants, but there appears to be a defect in the excision of pyrimidine dimers, allowing resealing via ligation at the site of the incision. The lack of NMN-stimulated repair synthesis in uvrC polA+ cells indicates that incision is not the only requirement for repair synthesis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYCE R. P., HOWARD-FLANDERS P. RELEASE OF ULTRAVIOLET LIGHT-INDUCED THYMINE DIMERS FROM DNA IN E. COLI K-12. Proc Natl Acad Sci U S A. 1964 Feb;51:293–300. doi: 10.1073/pnas.51.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ishai R., Sharon R. Patch size and base composition of ultraviolet light-induced repair synthesis in toluenized Escherichia coli. J Mol Biol. 1978 Apr 15;120(3):423–432. doi: 10.1016/0022-2836(78)90428-x. [DOI] [PubMed] [Google Scholar]
- Bowersock D., Moses R. E. Ultraviolet irradiation inhibition of replicative deoxyribonucleic acid synthesis in toluene-treated Escherichia coli. J Biol Chem. 1973 Nov 10;248(21):7449–7455. [PubMed] [Google Scholar]
- Braun A., Grossman L. An endonuclease from Escherichia coli that acts preferentially on UV-irradiated DNA and is absent from the uvrA and uvrB mutants. Proc Natl Acad Sci U S A. 1974 May;71(5):1838–1842. doi: 10.1073/pnas.71.5.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch W. A., Dorson J. W., Moses R. E. Excision of pyrimidine dimers in toluene-treated Escherichia coli. J Bacteriol. 1976 Jan;125(1):220–224. doi: 10.1128/jb.125.1.220-224.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorson J. W., Deutsch W. A., Moses R. E. Role of DNA polymerases in excision repair in Escherichia coli. J Biol Chem. 1978 Feb 10;253(3):660–664. [PubMed] [Google Scholar]
- Dorson J. W., Moses R. E. DNA polymerase I-mediated ultraviolet repair synthesis in toluene-treated Escherichia coli. J Biol Chem. 1978 Feb 10;253(3):665–670. [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Nash H. A. DNA gyrase: an enzyme that introduces superhelical turns into DNA. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3872–3876. doi: 10.1073/pnas.73.11.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman L., Braun A., Feldberg R., Mahler I. Enzymatic repair of DNA. Annu Rev Biochem. 1975;44:19–43. doi: 10.1146/annurev.bi.44.070175.000315. [DOI] [PubMed] [Google Scholar]
- Heijneker H. L., Ellens D. J., Tjeerde R. H., Glickman B. W., van Dorp B., Pouwels P. H. A mutant of Escherichia coli K12 deficient in the 5'-3' exonucleolytic activity of DNA polymerase I. II. Purification and properties of the mutant enzyme. Mol Gen Genet. 1973 Jul 31;124(1):83–96. doi: 10.1007/BF00267167. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P., Theriot L. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagen products from DNA. Genetics. 1966 Jun;53(6):1119–1136. doi: 10.1093/genetics/53.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T. Excision repair characteristics of recB - res - and uvrC - strains of Escherichia coli. J Bacteriol. 1972 Dec;112(3):1237–1246. doi: 10.1128/jb.112.3.1237-1246.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. B., Cozzarelli N. R., Deutscher M. P., Lehman I. R., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXXII. Replication of duplex deoxyribonucleic acid by polymerase at a single strand break. J Biol Chem. 1970 Jan 10;245(1):39–45. [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Little J. W., Zimmerman S. B., Oshinsky C. K., Gellert M. Enzymatic joining of DNA strands, II. An enzyme-adenylate intermediate in the dpn-dependent DNA ligase reaction. Proc Natl Acad Sci U S A. 1967 Nov;58(5):2004–2011. doi: 10.1073/pnas.58.5.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masamune Y., Richardson C. C. Strand displacement during deoxyribonucleic acid synthesis at single strand breaks. J Biol Chem. 1971 Apr 25;246(8):2692–2701. [PubMed] [Google Scholar]
- Masker W. E., Hanawalt P. C. Ultraviolet-stimulated DNA synthesis in toluenzied Escherichia coli deficient in DNA polymerase I. Proc Natl Acad Sci U S A. 1973 Jan;70(1):129–133. doi: 10.1073/pnas.70.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masker W. E., Simon T. J., Hanawalt P. C. Repair replication in permeabilized Escherichia coli. Basic Life Sci. 1975;5A:245–254. doi: 10.1007/978-1-4684-2895-7_32. [DOI] [PubMed] [Google Scholar]
- Masker W. E. The ATP dependence of the incision and resynthesis steps of excision repair. Biochim Biophys Acta. 1976 Aug 18;442(2):162–173. doi: 10.1016/0005-2787(76)90487-1. [DOI] [PubMed] [Google Scholar]
- McGrath R. A., Williams R. W. Reconstruction in vivo of irradiated Escherichia coli deoxyribonucleic acid; the rejoining of broken pieces. Nature. 1966 Oct 29;212(5061):534–535. doi: 10.1038/212534a0. [DOI] [PubMed] [Google Scholar]
- Monk M., Kinross J. Conditional lethality of recA and recB derivatives of a strain of Escherichia coli K-12 with a temperature-sensitive deoxyribonucleic acid polymerase I. J Bacteriol. 1972 Mar;109(3):971–978. doi: 10.1128/jb.109.3.971-978.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses R. E., Moody E. E. DNA repair synthesis dependent on the uvrA,B gene products in toluene-treated cells. J Biol Chem. 1975 Oct 25;250(20):8055–8061. [PubMed] [Google Scholar]
- Moses R. E., Richardson C. C. A new DNA polymerase acitvity of Escherichia coli. II. Properties of the enzyme purified from wild-type E. coli and DNA-ts mutants. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1565–1571. doi: 10.1016/0006-291x(70)90566-8. [DOI] [PubMed] [Google Scholar]
- Moses R. E., Richardson C. C. Replication and repair of DNA in cells of Escherichia coli treated with toluene. Proc Natl Acad Sci U S A. 1970 Oct;67(2):674–681. doi: 10.1073/pnas.67.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H., Shimada K., Tomizawa J. Studies on radiation-sensitive mutants of E. coli. I. Mutants defective in the repair synthesis. Mol Gen Genet. 1968 May 3;101(3):227–244. doi: 10.1007/BF00271625. [DOI] [PubMed] [Google Scholar]
- Otsuji N., Murayama I. Deoxyribonucleic acid damage by monofunctional mitomycins and its repair in Escherichia coli. J Bacteriol. 1972 Feb;109(2):475–483. doi: 10.1128/jb.109.2.475-483.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp W. D., Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968 Jan 28;31(2):291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- SETLOW R. B., CARRIER W. L. THE DISAPPEARANCE OF THYMINE DIMERS FROM DNA: AN ERROR-CORRECTING MECHANISM. Proc Natl Acad Sci U S A. 1964 Feb;51:226–231. doi: 10.1073/pnas.51.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneck P. K., Staudenbauer W. L. Escherichia coli DNA synthesis in vitro: insensitivity of ATP-dependent DNA repair to inhibition by novobiocin. Nucleic Acids Res. 1977 Jun;4(6):2057–2064. doi: 10.1093/nar/4.6.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeberg E., Nissen-Meyer J., Strike P. Incision of ultraviolet-irradiated DNA by extracts of E. coli requires three different gene products. Nature. 1976 Oct 7;263(5577):524–526. doi: 10.1038/263524a0. [DOI] [PubMed] [Google Scholar]
- Seeberg E. Reconstitution of an Escherichia coli repair endonuclease activity from the separated uvrA+ and uvrB+/uvrC+ gene products. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2569–2573. doi: 10.1073/pnas.75.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeberg E., Rupp W. D. Effect of mutations in lig and polA on UV-induced strand cutting in a uvrC strain of Escherichia coli. Basic Life Sci. 1975;5B:439–441. doi: 10.1007/978-1-4684-2898-8_3. [DOI] [PubMed] [Google Scholar]
- Seeberg E., Strike P. Excision repair of ultraviolet-irradiated deoxyribonucleic acid in plasmolyzed cells of Escherichia coli. J Bacteriol. 1976 Mar;125(3):787–795. doi: 10.1128/jb.125.3.787-795.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon R., Miller C., Ben-Ishai R. Two modes of excision repair in toluene-treated Escherichia coli. J Bacteriol. 1975 Sep;123(3):1107–1114. doi: 10.1128/jb.123.3.1107-1114.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Ogawa H., Tomizawa J. Studies on radiation-sensitive mutants of E. coli. II. Breakage and repair of ultraviolet irradiated intracellular DNA of phage lambda. Mol Gen Genet. 1968 May 3;101(3):245–256. doi: 10.1007/BF00271626. [DOI] [PubMed] [Google Scholar]
- Strike P. DNA synthesis and degradation in UV-irradiated toluene treated cells of E. coli K12: the role of polynucleotide ligase. Mol Gen Genet. 1977 Nov 29;157(1):99–107. doi: 10.1007/BF00268692. [DOI] [PubMed] [Google Scholar]
- Taketo A., Yasuda S., Sekiguchi M. Initial step of excision repair in Escherichia coli: replacement of defective function of uvr mutants by T4 endonuclease V. J Mol Biol. 1972 Sep 14;70(1):1–14. doi: 10.1016/0022-2836(72)90160-x. [DOI] [PubMed] [Google Scholar]
- Waldstein E. A., Sharon R., Ben-Ishai R. Role of ATP in excision repair of ultraviolet radiation damage in Escherichia coli. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2651–2654. doi: 10.1073/pnas.71.7.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



