Abstract
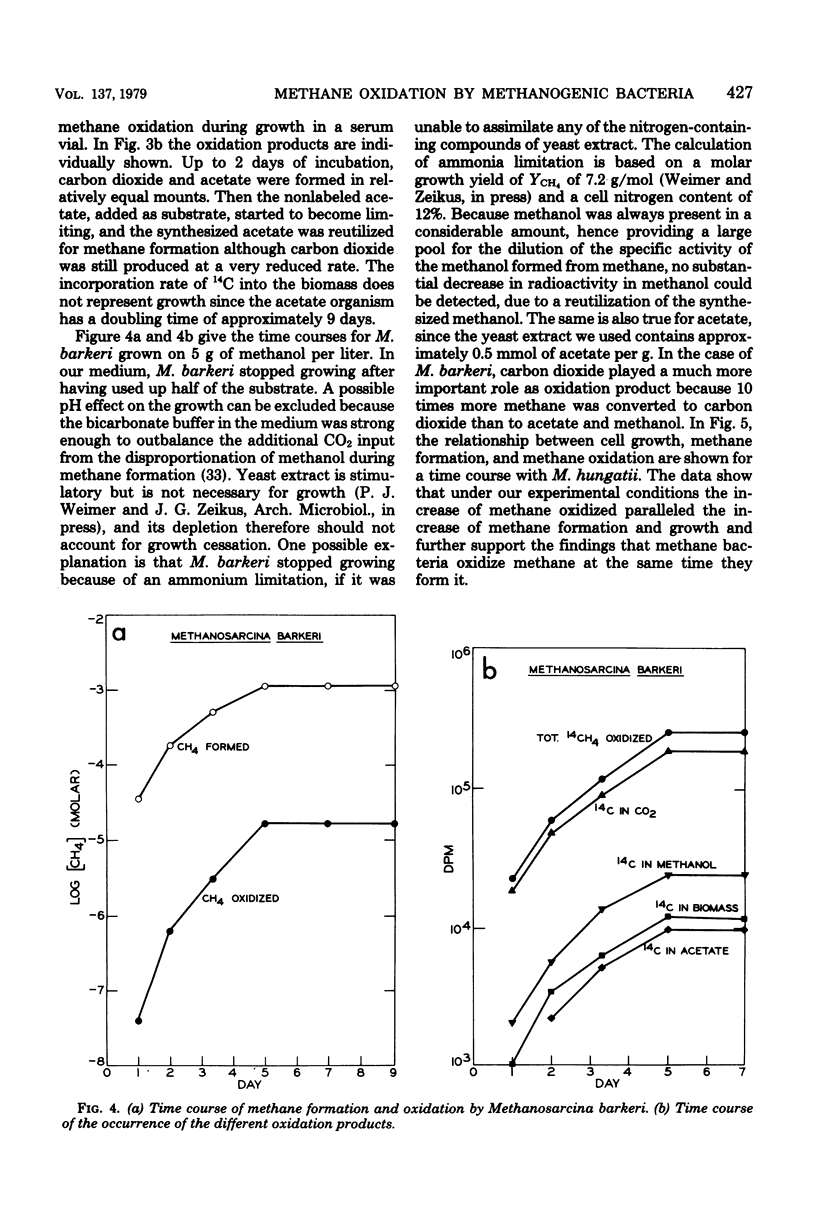
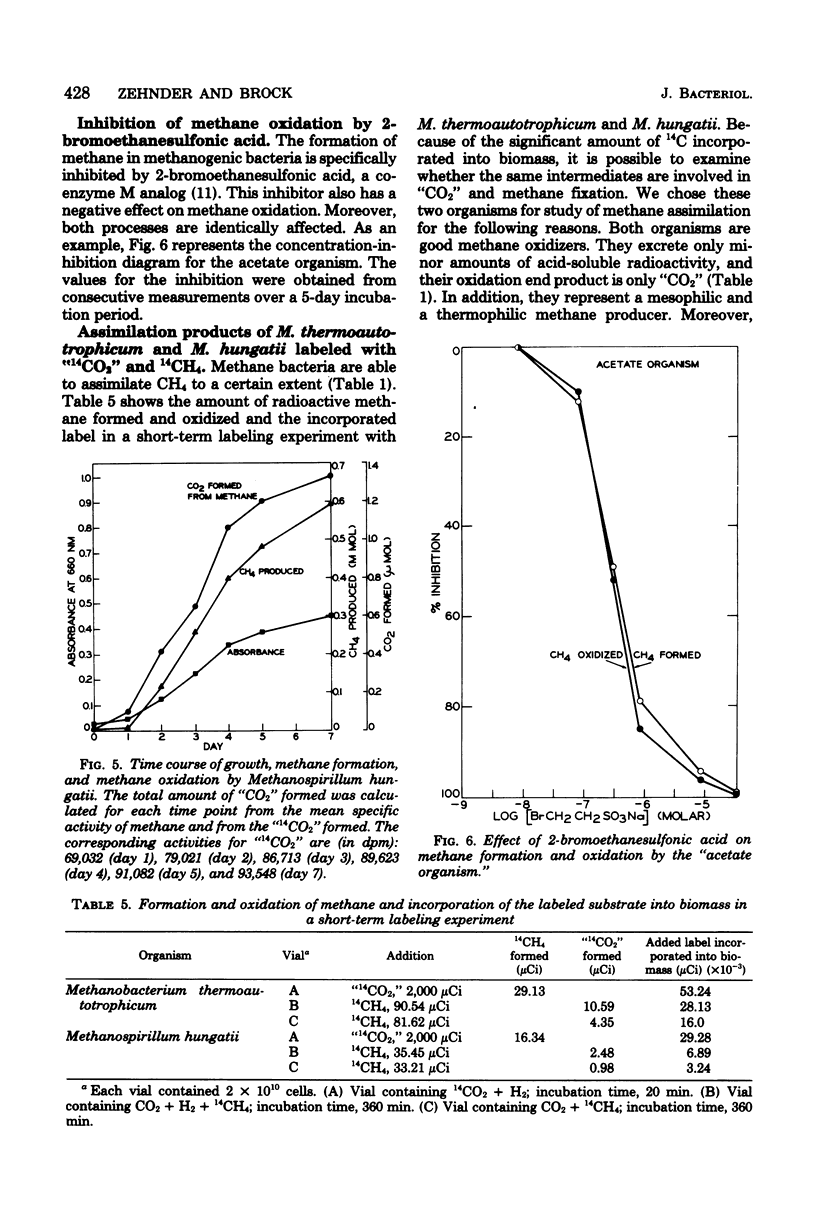
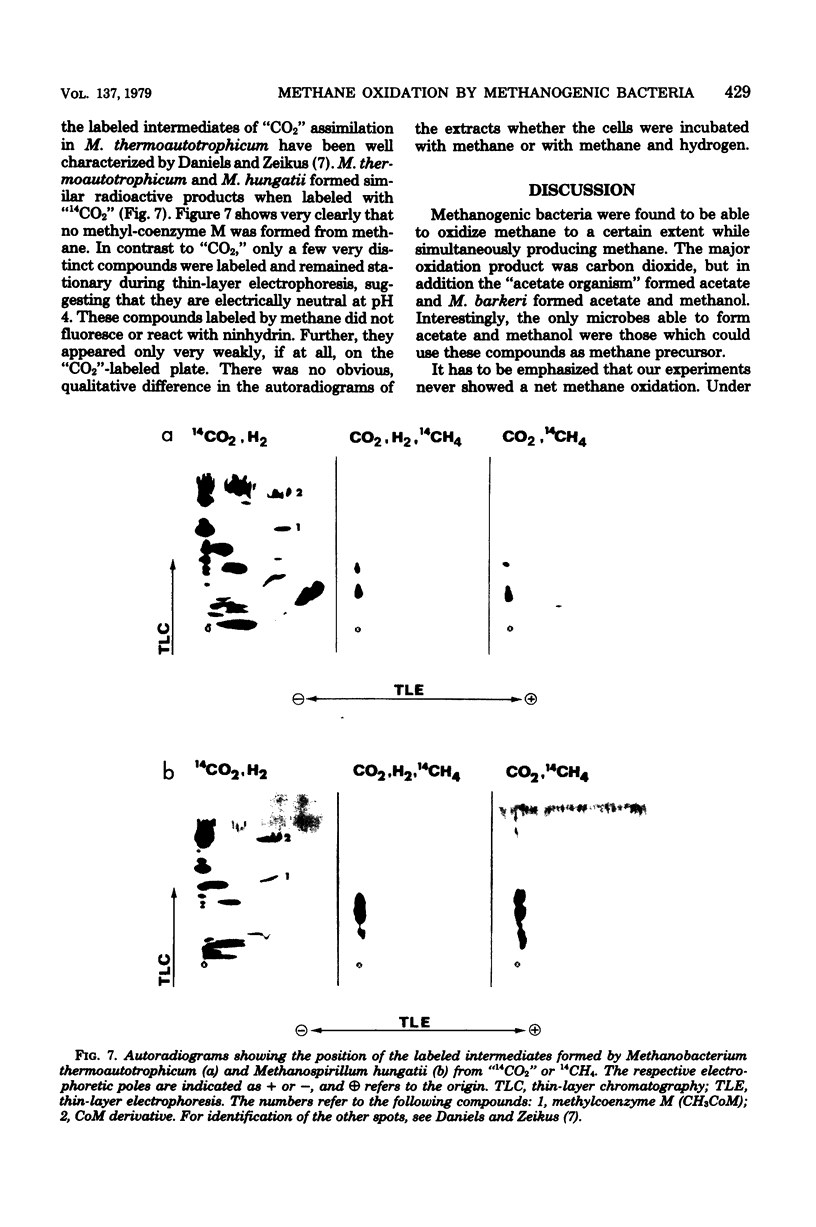
Methanogenic bacteria were found to form and oxidize methane at the same time. As compared to the quantity of methane formed, the amount of methane simultaneously oxidized varied between 0.3 and 0.001%, depending on the strain used. All the nine tested strains of methane producers (Methanobacterium ruminantium, Methanobacterium strain M.o.H., M. formicicum, M. thermoautotrophicum, M. arbophilicum, Methanobacterium strain AZ, Methanosarcina barkeri, Methanospirillum hungatii, and the "acetate organism") reoxidized methane to carbon dioxide. In addition, they assimilated a small part of the methane supplied into cell material. Methanol and acetate also occurred as oxidation products in M. barkeri cultures. Acetate was also formed by the "acetate organism," a methane bacterium unable to use methanogenic substrates other than acetate. Methane was the precursor of the methyl group of the acetate synthesized in the course of methane oxidation. Methane formation and its oxidation were inhibited equally by 2-bromoethanesulfonic acid. Short-term labeling experiments with M. thermoautotrophicum and M. hungatii clearly suggest that the pathway of methane oxidation is not identical with a simple back reaction of the methane formation process.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony C. The biochemistry of methylotrophic micro-organisms. Sci Prog. 1975 Summer;62(246):167–206. [PubMed] [Google Scholar]
- Balch W. E., Wolfe R. S. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressureized atmosphere. Appl Environ Microbiol. 1976 Dec;32(6):781–791. doi: 10.1128/aem.32.6.781-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P., Campbell L. L., Reddy C. A., Crabill M. R. Growth of desulfovibrio in lactate or ethanol media low in sulfate in association with H2-utilizing methanogenic bacteria. Appl Environ Microbiol. 1977 May;33(5):1162–1169. doi: 10.1128/aem.33.5.1162-1169.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P., Wolin E. A., Wolin M. J., Wolfe R. S. Methanobacillus omelianskii, a symbiotic association of two species of bacteria. Arch Mikrobiol. 1967;59(1):20–31. doi: 10.1007/BF00406313. [DOI] [PubMed] [Google Scholar]
- Daniels L., Zeikus J. G. One-carbon metabolism in methanogenic bacteria: analysis of short-term fixation products of 14CO2 and 14CH3OH incorporated into whole cells. J Bacteriol. 1978 Oct;136(1):75–84. doi: 10.1128/jb.136.1.75-84.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry J. G., Wolfe R. S. Anaerobic degradation of benzoate to methane by a microbial consortium. Arch Microbiol. 1976 Feb;107(1):33–40. doi: 10.1007/BF00427864. [DOI] [PubMed] [Google Scholar]
- Higgins I. J., Quayle J. R. Oxygenation of methane by methane-grown Pseudomonas methanica and Methanomonas methanooxidans. Biochem J. 1970 Jun;118(2):201–208. doi: 10.1042/bj1180201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaNoue K., Nicklas W. J., Williamson J. R. Control of citric acid cycle activity in rat heart mitochondria. J Biol Chem. 1970 Jan 10;245(1):102–111. [PubMed] [Google Scholar]
- MYLROIE R. L., HUNGATE R. E. Experiments on the methane bacteria in sludge. Can J Microbiol. 1954 Aug;1(1):55–64. doi: 10.1139/m55-008. [DOI] [PubMed] [Google Scholar]
- Nelson D. R., Zeikus J. G. Rapid method for the radioisotopic analysis of gaseous end products of anaerobic metabolism. Appl Microbiol. 1974 Aug;28(2):258–261. doi: 10.1128/am.28.2.258-261.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy C. A., Bryant M. P., Wolin M. J. Characteristics of S organism isolated from Methanobacillus omelianskii. J Bacteriol. 1972 Feb;109(2):539–545. doi: 10.1128/jb.109.2.539-545.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH P. H., HUNGATE R. E. Isolation and characterization of Methanobacterium ruminantium n. sp. J Bacteriol. 1958 Jun;75(6):713–718. doi: 10.1128/jb.75.6.713-718.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLIN E. A., WOLIN M. J., WOLFE R. S. FORMATION OF METHANE BY BACTERIAL EXTRACTS. J Biol Chem. 1963 Aug;238:2882–2886. [PubMed] [Google Scholar]
- Wertlieb D., Vishniac W. Methane utilization by a strain of Rhodopseudomonas gelatinosa. J Bacteriol. 1967 May;93(5):1722–1724. doi: 10.1128/jb.93.5.1722-1724.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdel F., Pfennig N. A new anaerobic, sporing, acetate-oxidizing, sulfate-reducing bacterium, Desulfotomaculum (emend.) acetoxidans. Arch Microbiol. 1977 Feb 4;112(1):119–122. doi: 10.1007/BF00446665. [DOI] [PubMed] [Google Scholar]
- Zehnder A. J., Wuhrmann K. Titanium (III) citrate as a nontoxic oxidation-reduction buffering system for the culture of obligate anaerobes. Science. 1976 Dec 10;194(4270):1165–1166. doi: 10.1126/science.793008. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G., Henning D. L. Methanobacterium arbophilicum sp.nov. An obligate anaerobe isolated from wetwood of living trees. Antonie Van Leeuwenhoek. 1975;41(4):543–552. doi: 10.1007/BF02565096. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G. The biology of methanogenic bacteria. Bacteriol Rev. 1977 Jun;41(2):514–541. doi: 10.1128/br.41.2.514-541.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Wolfe R. S. Methanobacterium thermoautotrophicus sp. n., an anaerobic, autotrophic, extreme thermophile. J Bacteriol. 1972 Feb;109(2):707–715. doi: 10.1128/jb.109.2.707-713.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg L., Patel G. B., Clark D. S., Lentz C. P. Factors affecting rate of methane formation from acetic acid by enriched methanogenic cultures. Can J Microbiol. 1976 Sep;22(9):1312–1319. doi: 10.1139/m76-194. [DOI] [PubMed] [Google Scholar]